Differences in glutamate uptake between cortical regions impact neuronal NMDA receptor activation
- PMID: 30963115
- PMCID: PMC6451009
- DOI: 10.1038/s42003-019-0367-9
Differences in glutamate uptake between cortical regions impact neuronal NMDA receptor activation
Abstract
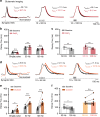
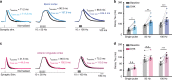
Removal of synaptically-released glutamate by astrocytes is necessary to spatially and temporally limit neuronal activation. Recent evidence suggests that astrocytes may have specialized functions in specific circuits, but the extent and significance of such specialization are unclear. By performing direct patch-clamp recordings and two-photon glutamate imaging, we report that in the somatosensory cortex, glutamate uptake by astrocytes is slower during sustained synaptic stimulation when compared to lower stimulation frequencies. Conversely, glutamate uptake capacity is increased in the frontal cortex during higher frequency synaptic stimulation, thereby limiting extracellular buildup of glutamate and NMDA receptor activation in layer 5 pyramidal neurons. This efficient glutamate clearance relies on Na+/K+-ATPase function and both GLT-1 and non-GLT-1 transporters. Thus, by enhancing their glutamate uptake capacity, astrocytes in the frontal cortex may prevent excessive neuronal excitation during intense synaptic activity. These results may explain why diseases associated with network hyperexcitability differentially affect individual brain areas.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

