Extracellular and intracellular sphingosine-1-phosphate distinctly regulates exocytosis in chromaffin cells
- PMID: 30963576
- PMCID: PMC6591091
- DOI: 10.1111/jnc.14703
Extracellular and intracellular sphingosine-1-phosphate distinctly regulates exocytosis in chromaffin cells
Abstract
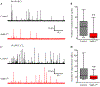
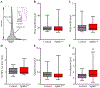
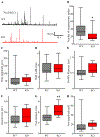
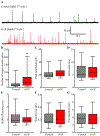
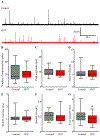
Sphingosine-1-phosphate (S1P) is an essential bioactive sphingosine lipid involved in many neurological disorders. Sphingosine kinase 1 (SphK1), a key enzyme for S1P production, is concentrated in presynaptic terminals. However, the role of S1P/SphK1 signaling in exocytosis remains elusive. By detecting catecholamine release from single vesicles in chromaffin cells, we show that a dominant negative SphK1 (SphK1DN ) reduces the number of amperometric spikes and increases the duration of foot, which reflects release through a fusion pore, implying critical roles for S1P in regulating the rate of exocytosis and fusion pore expansion. Similar phenotypes were observed in chromaffin cells obtained from SphK1 knockout mice compared to those from wild-type mice. In addition, extracellular S1P treatment increased the number of amperometric spikes, and this increase, in turn, was inhibited by a selective S1P3 receptor blocker, suggesting extracellular S1P may regulate the rate of exocytosis via activation of S1P3. Furthermore, intracellular S1P application induced a decrease in foot duration of amperometric spikes in control cells, indicating intracellular S1P may regulate fusion pore expansion during exocytosis. Taken together, our study represents the first demonstration that S1P regulates exocytosis through distinct mechanisms: extracellular S1P may modulate the rate of exocytosis via activation of S1P receptors while intracellular S1P may directly control fusion pore expansion during exocytosis. OPEN SCIENCE BADGES: This article has received a badge for *Open Materials* because it provided all relevant information to reproduce the study in the manuscript. The complete Open Science Disclosure form for this article can be found at the end of the article. More information about the Open Practices badges can be found at https://cos.io/our-services/open-science-badges/.
Keywords: amperometry; chromaffin cell; exocytosis; fusion; sphingosine kinase 1; sphingosine-1-phosphate.
© 2019 International Society for Neurochemistry.
Conflict of interest statement
Figures
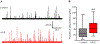

References
-
- Allende ML, Sasaki T, Kawai H et al. (2004) Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. The Journal of biological chemistry, 279, 52487–52492. - PubMed
-
- Alvarez SE, Milstien S and Spiegel S (2007) Autocrine and paracrine roles of sphingosine-1-phosphate. Trends in endocrinology and metabolism: TEM, 18, 300–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

