Survival of retinal ganglion cells after damage to the occipital lobe in humans is activity dependent
- PMID: 30963844
- PMCID: PMC6408898
- DOI: 10.1098/rspb.2018.2733
Survival of retinal ganglion cells after damage to the occipital lobe in humans is activity dependent
Abstract
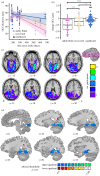
Damage to the optic radiations or primary visual cortex leads to blindness in all or part of the contralesional visual field. Such damage disconnects the retina from its downstream targets and, over time, leads to trans-synaptic retrograde degeneration of retinal ganglion cells. To date, visual ability is the only predictor of retinal ganglion cell degeneration that has been investigated after geniculostriate damage. Given prior findings that some patients have preserved visual cortex activity for stimuli presented in their blind field, we tested whether that activity explains variability in retinal ganglion cell degeneration over and above visual ability. We prospectively studied 15 patients (four females, mean age = 63.7 years) with homonymous visual field defects secondary to stroke, 10 of whom were tested within the first two months after stroke. Each patient completed automated Humphrey visual field testing, retinotopic mapping with functional magnetic resonance imaging, and spectral-domain optical coherence tomography of the macula. There was a positive relation between ganglion cell complex (GCC) thickness in the blind field and early visual cortex activity for stimuli presented in the blind field. Furthermore, residual visual cortex activity for stimuli presented in the blind field soon after the stroke predicted the degree of retinal GCC thinning six months later. These findings indicate that retinal ganglion cell survival after ischaemic damage to the geniculostriate pathway is activity dependent.
Keywords: functional magnetic resonance imaging; homonymous hemianopia; optical coherence tomography; retinal ganglion cells; retinotopic mapping; stroke.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Sectoral analysis of the retinal nerve fiber layer thinning and its association with visual field loss in homonymous hemianopia caused by post-geniculate lesions using spectral-domain optical coherence tomography.Graefes Arch Clin Exp Ophthalmol. 2016 Apr;254(4):745-56. doi: 10.1007/s00417-015-3181-1. Epub 2015 Oct 7. Graefes Arch Clin Exp Ophthalmol. 2016. PMID: 26446718 Free PMC article.
-
Transsynaptic Ganglion Cell Degeneration in Adult Patients After Occipital Lobe Stroke.J Neuroophthalmol. 2023 Jun 1;43(2):243-247. doi: 10.1097/WNO.0000000000001657. Epub 2022 Jun 23. J Neuroophthalmol. 2023. PMID: 35763809
-
Quantification of retinal ganglion cell loss in patients with homonymous visual field defect due to stroke.Neurol Sci. 2023 Aug;44(8):2811-2819. doi: 10.1007/s10072-023-06675-2. Epub 2023 Mar 11. Neurol Sci. 2023. PMID: 36905449
-
Trans-synaptic Retrograde Degeneration in the Human Visual System: Slow, Silent, and Real.Curr Neurol Neurosci Rep. 2017 Feb;17(2):16. doi: 10.1007/s11910-017-0725-2. Curr Neurol Neurosci Rep. 2017. PMID: 28229400 Review.
-
Visual fields and optical coherence tomography (OCT) in neuro-ophthalmology: Structure-function correlation.J Neurol Sci. 2021 Oct 15;429:118064. doi: 10.1016/j.jns.2021.118064. Epub 2021 Sep 1. J Neurol Sci. 2021. PMID: 34488042 Review.
Cited by
-
Visual Field Reconstruction in Hemianopia Using fMRI Based Mapping Techniques.Front Hum Neurosci. 2021 Aug 10;15:713114. doi: 10.3389/fnhum.2021.713114. eCollection 2021. Front Hum Neurosci. 2021. PMID: 34447301 Free PMC article.
-
Empty Sella Syndrome as a Window Into the Neuroprotective Effects of Prolactin.Front Med (Lausanne). 2021 Jul 8;8:680602. doi: 10.3389/fmed.2021.680602. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34307410 Free PMC article.
-
Evolution of Visual Field Defects After Occipital Stroke: A Quantitative Analysis.Transl Vis Sci Technol. 2025 Jun 2;14(6):14. doi: 10.1167/tvst.14.6.14. Transl Vis Sci Technol. 2025. PMID: 40478590 Free PMC article.
-
Training in cortically-blind fields confers patient-specific benefit against retinal thinning after occipital stroke.medRxiv [Preprint]. 2023 Dec 20:2023.12.19.23298260. doi: 10.1101/2023.12.19.23298260. medRxiv. 2023. Update in: Invest Ophthalmol Vis Sci. 2024 Apr 1;65(4):29. doi: 10.1167/iovs.65.4.29. PMID: 38196617 Free PMC article. Updated. Preprint.
-
Training in Cortically Blinded Fields Appears to Confer Patient-Specific Benefit Against Retinal Thinning.Invest Ophthalmol Vis Sci. 2024 Apr 1;65(4):29. doi: 10.1167/iovs.65.4.29. Invest Ophthalmol Vis Sci. 2024. PMID: 38635245 Free PMC article.
References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

