Biocompatible biodegradable polycaprolactone/basil seed mucilage scaffold for cell culture
- PMID: 30964022
- PMCID: PMC8676121
- DOI: 10.1049/iet-nbt.2018.5071
Biocompatible biodegradable polycaprolactone/basil seed mucilage scaffold for cell culture
Abstract
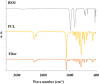
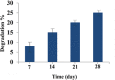
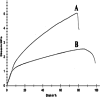
In this study, a polymer obtained from the basil seed mucilage (BSM) in combination with polycaprolactone (PCL), was used in the 2D scaffold production process for cell culture. First, combinations of two polymers with different ratios and concentrations were prepared and electrospun. Among these samples, a sample with a BSM/PCL ratio of 2/3 was used to perform different tests due to its fibre uniformity and appropriate diameter. The Fourier transform infrared spectrometer test was carried out to chemically analyse the scaffold, the X-ray diffraction test to determine the crystallinity of the scaffold, and the contact angle test to determine the hydrophilicity of the scaffold. The strength, porosity, and degradation percentage of the scaffold were also studied. With appropriate conditions of the scaffold for cell culture determined, Vero epithelial cells were cultured on the scaffold. Results obtained from cell culture indicated that the adhesion of the scaffold was suitable for the appropriate growth cells.
Figures




References
-
- Temenoff J.S. Mikos A.G.: ‘Review: tissue engineering for regeneration of articular cartilage’, Biomaterials, 2000, 21, pp. 431 –440 - PubMed
-
- Murugan R. Ramakrishna S.: ‘Design strategies of tissue engineering scaffolds with controlled fiber orientation’, Tissue Eng., 2007, 13, pp. 1845 –1866 - PubMed
-
- Huang Zh.‐M. Zhang Y.‐Z. Kotak M. et al.: ‘A review on polymer nanofibers by electrospinning and their applications in nanocomposites’, Compos. Sci. Technol., 2003, 63, pp. 2223 –2253
-
- Chavan S. S. Sinha M. K. Londhe P.V.: ‘Synthesis and characterization of composite nanofibers with VARTM and electrospinning process’, Carbon Sci. Technol., 2013, 5, pp. 289 –295
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

