Role of macrophages in peripheral nerve injury and repair
- PMID: 30964051
- PMCID: PMC6524518
- DOI: 10.4103/1673-5374.253510
Role of macrophages in peripheral nerve injury and repair
Abstract
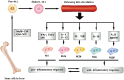
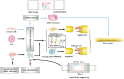
Resident and inflammatory macrophages are essential effectors of the innate immune system. These cells provide innate immune defenses and regulate tissue and organ homeostasis. In addition to their roles in diseases such as cancer, obesity and osteoarthritis, they play vital roles in tissue repair and disease rehabilitation. Macrophages and other inflammatory cells are recruited to tissue injury sites where they promote changes in the microenvironment. Among the inflammatory cell types, only macrophages have both pro-inflammatory (M1) and anti-inflammatory (M2) actions, and M2 macrophages have four subtypes. The co-action of M1 and M2 subtypes can create a favorable microenvironment, releasing cytokines for damaged tissue repair. In this review, we discuss the activation of macrophages and their roles in severe peripheral nerve injury. We also describe the therapeutic potential of macrophages in nerve tissue engineering treatment and highlight approaches for enhancing M2 cell-mediated nerve repair and regeneration.
Keywords: function; macrophage; nerve injury; nerve regeneration; nerve repair; neural regeneration; origin; polarization; tissue engineering.
Conflict of interest statement
None
Figures
Similar articles
-
Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury.Acta Neuropathol. 2015 Nov;130(5):605-18. doi: 10.1007/s00401-015-1482-4. Epub 2015 Sep 29. Acta Neuropathol. 2015. PMID: 26419777 Review.
-
Autologous Platelet-Rich Growth Factor Reduces M1 Macrophages and Modulates Inflammatory Microenvironments to Promote Sciatic Nerve Regeneration.Biomedicines. 2022 Aug 17;10(8):1991. doi: 10.3390/biomedicines10081991. Biomedicines. 2022. PMID: 36009539 Free PMC article.
-
Changes of pro-inflammatory and anti-inflammatory macrophages after peripheral nerve injury.RSC Adv. 2020 Oct 22;10(64):38767-38773. doi: 10.1039/d0ra06607a. eCollection 2020 Oct 21. RSC Adv. 2020. PMID: 35518415 Free PMC article.
-
Dynamic impact of brief electrical nerve stimulation on the neural immune axis-polarization of macrophages toward a pro-repair phenotype in demyelinated peripheral nerve.Glia. 2016 Sep;64(9):1546-61. doi: 10.1002/glia.23021. Epub 2016 Jun 29. Glia. 2016. PMID: 27353566
-
Hydrogel-based immunoregulation of macrophages for tissue repair and regeneration.Int J Biol Macromol. 2024 May;268(Pt 1):131643. doi: 10.1016/j.ijbiomac.2024.131643. Epub 2024 Apr 21. Int J Biol Macromol. 2024. PMID: 38643918 Review.
Cited by
-
Progress in the application of sustained-release drug microspheres in tissue engineering.Mater Today Bio. 2022 Aug 13;16:100394. doi: 10.1016/j.mtbio.2022.100394. eCollection 2022 Dec. Mater Today Bio. 2022. PMID: 36042853 Free PMC article. Review.
-
The importance of N6-methyladenosine modification in tumor immunity and immunotherapy.Exp Hematol Oncol. 2022 May 19;11(1):30. doi: 10.1186/s40164-022-00281-2. Exp Hematol Oncol. 2022. PMID: 35590394 Free PMC article. Review.
-
Strychnos nux-vomica L. seed preparation promotes functional recovery and attenuates oxidative stress in a mouse model of sciatic nerve crush injury.BMC Complement Med Ther. 2020 Jun 11;20(1):181. doi: 10.1186/s12906-020-02950-3. BMC Complement Med Ther. 2020. PMID: 32527244 Free PMC article.
-
Macrophage-Neuroglia Interactions in Promoting Neuronal Regeneration in Zebrafish.Int J Mol Sci. 2023 Mar 30;24(7):6483. doi: 10.3390/ijms24076483. Int J Mol Sci. 2023. PMID: 37047456 Free PMC article. Review.
-
Development and in vitro evaluation of biomimetic injectable hydrogels from decellularized human nerves for central nervous system regeneration.Mater Today Bio. 2025 Jan 11;31:101483. doi: 10.1016/j.mtbio.2025.101483. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 39896276 Free PMC article.
References
-
- Armstrong BD, Hu Z, Abad C, Yamamoto M, Rodriguez WI, Cheng J, Tam J, Gomariz RP, Patterson PH, Waschek JA. Lymphocyte regulation of neuropeptide gene expression after neuronal injury. J Neurosci Res. 2003;74:240–247. - PubMed
-
- Binder NB, Niederreiter B, Hoffmann O, Stange R, Pap T, Stulnig TM, Mack M, Erben RG, Smolen JS, Redlich K. Estrogen-dependent and C-C chemokine receptor-2-dependent pathways determine osteoclast behavior in osteoporosis. Nat Med. 2009;15:417–424. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

