Transient Introduction of miR-294 in the Heart Promotes Cardiomyocyte Cell Cycle Reentry After Injury
- PMID: 30964391
- PMCID: PMC6586499
- DOI: 10.1161/CIRCRESAHA.118.314223
Transient Introduction of miR-294 in the Heart Promotes Cardiomyocyte Cell Cycle Reentry After Injury
Abstract
Rationale: Embryonic heart is characterized of rapidly dividing cardiomyocytes required to build a working myocardium. Cardiomyocytes retain some proliferative capacity in the neonates but lose it in adulthood. Consequently, a number of signaling hubs including microRNAs are altered during cardiac development that adversely impacts regenerative potential of cardiac tissue. Embryonic stem cell cycle miRs are a class of microRNAs exclusively expressed during developmental stages; however, their effect on cardiomyocyte proliferation and heart function in adult myocardium has not been studied previously.
Objective: To determine whether transient reintroduction of embryonic stem cell cycle miR-294 promotes cardiomyocyte cell cycle reentry enhancing cardiac repair after myocardial injury.
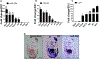
Methods and results: miR-294 is expressed in the heart during development, prenatal stages, lost in the neonate, and adult heart confirmed by qRT-PCR and in situ hybridization. Neonatal ventricular myocytes treated with miR-294 showed elevated expression of Ki67, p-histone H3, and Aurora B confirmed by immunocytochemistry compared with control cells. miR-294 enhanced oxidative phosphorylation and glycolysis in Neonatal ventricular myocytes measured by seahorse assay. Mechanistically, miR-294 represses Wee1 leading to increased activity of the cyclin B1/CDK1 complex confirmed by qRT-PCR and immunoblot analysis. Next, a doxycycline-inducible AAV9-miR-294 vector was delivered to mice for activating miR-294 in myocytes for 14 days continuously after myocardial infarction. miR-294-treated mice significantly improved left ventricular functions together with decreased infarct size and apoptosis 8 weeks after MI. Myocyte cell cycle reentry increased in miR-294 hearts analyzed by Ki67, pH3, and AurB (Aurora B kinase) expression parallel to increased small myocyte number in the heart. Isolated adult myocytes from miR-294 hearts showed increased 5-ethynyl-2'-deoxyuridine+ cells and upregulation of cell cycle markers and miR-294 targets 8 weeks after MI.
Conclusions: Ectopic transient expression of miR-294 recapitulates developmental signaling and phenotype in cardiomyocytes promoting cell cycle reentry that leads to augmented cardiac function in mice after myocardial infarction.
Keywords: cell cycle; embryonic stem cells; microRNAs; myocardial infarction; myocardium.
Figures

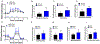





Comment in
-
Embryonic miRs to the Rescue.Circ Res. 2019 Jun 21;125(1):26-28. doi: 10.1161/CIRCRESAHA.119.315284. Epub 2019 Jun 20. Circ Res. 2019. PMID: 31219747 No abstract available.
References
-
- Chen JF, Murchison EP, Tang R, Callis TE, Tatsuguchi M, Deng Z, Rojas M, Hammond SM, Schneider MD, Selzman CH, Meissner G, Patterson C, Hannon GJ and Wang DZ. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc Natl Acad Sci U S A. 2008;105:2111–6. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

