Glucocorticosteroids for people with alcoholic hepatitis
- PMID: 30964545
- PMCID: PMC6455893
- DOI: 10.1002/14651858.CD001511.pub4
Glucocorticosteroids for people with alcoholic hepatitis
Abstract
Background: Alcoholic hepatitis is a form of alcoholic liver disease characterised by steatosis, necroinflammation, fibrosis, and complications to the liver. Typically, alcoholic hepatitis presents in people between 40 and 50 years of age. Alcoholic hepatitis can be resolved if people abstain from drinking, but the risk of death will depend on the severity of the liver damage and abstinence from alcohol. Glucocorticosteroids have been studied extensively in randomised clinical trials to assess their benefits and harms. However, the results have been contradictory.
Objectives: To assess the benefits and harms of glucocorticosteroids in people with alcoholic hepatitis.
Search methods: We identified trials through electronic searches in Cochrane Hepato-Biliary's (CHB) Controlled Trials Register, CENTRAL, MEDLINE, Embase, LILACS, and Science Citation Index Expanded. We looked for ongoing or unpublished trials in clinical trials registers and pharmaceutical company sources. We also scanned reference lists of the studies retrieved. The last search was 18 January 2019.
Selection criteria: Randomised clinical trials assessing glucocorticosteroids versus placebo or no intervention in people with alcoholic hepatitis, irrespective of year, language of publication, or format. We considered trials with adults diagnosed with alcoholic hepatitis, which could have been established through clinical or biochemical diagnostic criteria or both. We defined alcoholic hepatitis as mild (Maddrey's score less than 32) and severe (Maddrey's score 32 or more). We allowed cointerventions in the trial groups, provided they were similar.
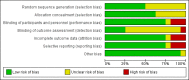
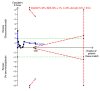
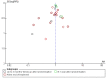
Data collection and analysis: We followed Cochrane methodology, performing the meta-analyses using Review Manager 5. We presented the results of dichotomous outcomes as risk ratios (RR) and of continuous outcomes as mean difference (MD), with 95% confidence intervals (CI). We used both the fixed-effect and the random-effects models for meta-analyses. Whenever there were significant discrepancies in the results, we reported the more conservative point estimate of the two. We considered a P value of 0.01 or less, two-tailed, as statistically significant if the required information size was reached for our three primary outcomes (all-cause mortality, health-related quality of life, and serious adverse events during treatment) and our post hoc decision to include analyses of mortality at more time points. We presented heterogeneity using the I² statistic. If trialists used intention-to-treat analysis to deal with missing data, we used these data in our primary analysis; otherwise, we used the available data. We assessed the bias risk of the trials using bias risk domains and the certainty of the evidence using GRADE.
Main results: Sixteen trials fulfilled our inclusion criteria. All trials but one were at overall high risk of bias. Fifteen trials (one of which was an abstract) provided data for analysis (927 participants received glucocorticosteroids and 934 participants received placebo or no intervention). Glucocorticosteroids were administered orally or parenterally for a median 28 days (range 3 days to 12 weeks). The participants were between 25 and 70 years old, had different stages of alcoholic liver disease, and 65% were men. Follow-up, when reported, was up to the moment of discharge from the hospital, until they died (median of 63 days), or for at least one year. There was no evidence of effect of glucocorticosteroids on all-cause mortality up to three months following randomisation (random-effects RR 0.90, 95% CI 0.70 to 1.15; participants = 1861; trials = 15; very low-certainty evidence) or on health-related quality of life up to three months, measured with the European Quality of Life - 5 Dimensions - 3 Levels scale (MD -0.04 points, 95% CI -0.11 to 0.03; participants = 377; trial = 1; low-certainty evidence). There was no evidence of effect on the occurrence of serious adverse events during treatment (random-effects RR 1.05, 95% CI 0.85 to 1.29; participants = 1861; trials = 15; very low-certainty evidence), liver-related mortality up to three months following randomisation (random-effects RR 0.89, 95% CI 0.69 to 1.14; participants = 1861; trials = 15; very low-certainty evidence), number of participants with any complications up to three months following randomisation (random-effects RR 1.04, 95% CI 0.86 to 1.27; participants = 1861; very low-certainty evidence), and number of participants of non-serious adverse events up to three months' follow-up after end of treatment (random-effects RR 1.99, 95% CI 0.72 to 5.48; participants = 160; trials = 4; very low-certainty evidence). Based on the information that we collected from the published trial reports, only one of the trials seems not to be industry-funded, and the remaining 15 trials did not report clearly whether they were partly or completely funded by the industry.
Authors' conclusions: We are very uncertain about the effect estimate of no difference between glucocorticosteroids and placebo or no intervention on all-cause mortality and serious adverse events during treatment because the certainty of evidence was very low, and low for health-related quality of life. Due to inadequate reporting, we cannot exclude increases in adverse events. As the CIs were wide, we cannot rule out significant benefits or harms of glucocorticosteroids. Therefore, we need placebo-controlled randomised clinical trials, designed according to the SPIRIT guidelines and reported according to the CONSORT guidelines. Future trials ought to report depersonalised individual participant data, so that proper individual participant data meta-analyses of the effects of glucocorticosteroids in subgroups can be conducted.
Conflict of interest statement
CP: none. DV: none. GC: none. ET: none. DN: none. CG: none.
Figures

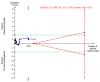
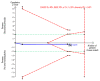
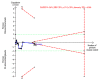
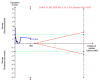
Update of
-
Glucocorticosteroids for people with alcoholic hepatitis.Cochrane Database Syst Rev. 2017 Nov 2;11(11):CD001511. doi: 10.1002/14651858.CD001511.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2019 Apr 09;4:CD001511. doi: 10.1002/14651858.CD001511.pub4. PMID: 29096421 Free PMC article. Updated.
References
References to studies included in this review
Blitzer 1977 {published data only}
-
- Blitzer BL, Mutchnick MG, Joshi PH, Phillips MM, Fessel JM, Conn HO. Adrenocorticosteroid therapy in alcoholic hepatitis. A prospective, double‐blind randomized study. American Journal of Digestive Diseases 1977;22(6):477‐84. [MEDLINE: ] - PubMed
-
- Blitzer BL, Mutchnick MG, Joshi PH, Phillips MM, Fessel JM, Conn HO. Adrenocorticosteroid therapy in alcoholic hepatitis. A prospective, double‐blind randomized study. Gastroenterology 1973;64:880. - PubMed
Bories 1987 {published data only}
-
- Bories P, Guedj JY, Mirouze D, Yousfi A, Michel H. Prednisolone treatment of alcoholic hepatitis: forty‐five patients [Traitement de l'hepatite alcoolique aigue par la prednisolone. Quarante‐cinq malades]. La Presse Medicale 1987;16:769‐72. [MEDLINE: ] - PubMed
Campra 1973 {published data only}
-
- Campra JL, Hamlin EM Jr, Kirshbaum RJ, Olivier M, Redeker AG, Reynolds TB. Prednisone therapy of acute alcoholic hepatitis. Report of a controlled trial. Annals of Internal Medicine 1973;79(5):625‐31. [MEDLINE: ] - PubMed
Carithers 1989 {published data only}
-
- Erratum: methylprednisolone therapy in patients with severe alcoholic hepatitis: randomized multicenter trial (American Journal of Gastroenterology, Vol. 85, No. 4 (473)). American Journal of Gastroenterology 1990; Vol. 85, issue 6:776. [CRS: 6800131000045704; EMBASE: 1990185566]
-
- Carithers RJ, Herlong HF, Diehl AM, Shaw EW, Combes B, Fallon HJ, et al. Corticosteroid therapy of alcoholic hepatitis: how many studies will it take?. Hepatology (Baltimore, Md.) 1990;12(3):619‐20. [CENTRAL: 844269; CRS: 6800100000024896; CN‐00844269] - PubMed
-
- Carithers RL Jr, Herlong HF, Diehl AM, Shaw EW, Combes B, Fallon HJ, et al. Methylprednisolone therapy in patients with severe alcoholic hepatitis. A randomized multicenter trial. Annals of Internal Medicine 1989;110:685‐90. [MEDLINE: ] - PubMed
-
- Maddrey WC, Carithers R Jr, Herlong HF, Combes B, Diehl AM, Shaw E, et al. Prednisolone therapy in patients with severe alcoholic hepatitis: results of a multicenter trial. Hepatology (Baltimore, Md.) 1986;6(5):1202.
De 2014 {published data only}
Depew 1980 {published data only}
-
- Depew W, Boyer T, Omata M, Redeker A, Reynolds T. Double‐blind controlled trial of prednisolone therapy in patients with severe acute alcoholic hepatitis and spontaneous encephalopathy. Gastroenterology 1980;78(3):524‐9. [MEDLINE: ] - PubMed
-
- Depew WT, Boyer TD, Omata M, Redeker AG, Reynolds TB. Double‐blind controlled trial of corticosteroid‐therapy in severe alcoholic hepatitis with encephalopathy. Clinical Research 1978;26(2):A150. [CRS: 6800131000045795]
Helman 1971 {published data only}
-
- Helman RA, Temko MH, Nye SW, Fallon HJ. Alcoholic hepatitis. Natural history and evaluation of prednisolone therapy. Annals of Internal Medicine 1971;74(3):311‐21. [MEDLINE: ] - PubMed
Maddrey 1978 {published data only}
-
- Maddrey WC, Boitnott JK, Bedine MS. Corticosteroid treatment of alcoholic liver disease: a controlled trial. Gastroenterology 1977;72(5 II):A‐148. [CRS: 6800131000045726; EMBASE: 0978117751]
-
- Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978;75(2):193‐9. [MEDLINE: ] - PubMed
Mendenhall 1977 {published data only}
-
- Mendenhall CL, Goldberg S. Risk factors and therapy in alcoholic hepatitis (AH). Gastroenterology 1977;72(5):1100.
Mendenhall 1984 {published data only}
-
- Mendenhall C, The Cooperative Study on Alcoholic Hepatitis. Survival after steroid treatment (T) for alcoholic hepatitis (AH). Hepatology (Baltimore, Md.) 1983;3(5):850. [CENTRAL: 221691; CRS: 6800100000005197; CN‐00221691]
-
- Mendenhall CL, Anderson S, Garcia‐Pont P, Goldberg S, Kiernan T, Seeff LB, et al. Short‐term and long‐term survival in patients with alcoholic hepatitis treated with oxandrolone and prednisolone. New England Journal of Medicine 1984;311(23):1464‐70. [MEDLINE: ] - PubMed
-
- Mendenhall CL, Roselle GA, Gartside P, Moritz T. Relationship of protein calorie malnutrition to alcoholic liver disease: a reexamination of data from two Veterans Administration Cooperative Studies. Alcoholism, Clinical and Experimental Research 1995;19(3):635‐41. [MEDLINE: ] - PubMed
Porter 1971 {published data only}
-
- Porter HP, Simon FR, Pope CE II, Volwiler W, Fenster LF. Corticosteroid therapy in severe alcoholic hepatitis. A double‐blind drug trial. New England Journal of Medicine 1971;284(24):1350‐5. [MEDLINE: ] - PubMed
Ramond 1992 {published data only}
-
- Mathurin P, Duchatelle V, Ramond MJ, Degott C, Bedossa P, Erlinger S, et al. Long term survival and prognostic factors in patients with severe biopsy‐proved alcoholic hepatitis (AH) treated by prednisolone: randomized trial, new cohort and simulation. Hepatology (Baltimore, Md.) 1994;20(4):319A.
-
- Mathurin P, Duchatelle V, Ramond MJ, Degott C, Bedossa P, Erlinger S, et al. Survival and prognostic factors in patients with severe alcoholic hepatitis treated with prednisolone. Gastroenterology 1996;110(6):1847‐53. [MEDLINE: ] - PubMed
-
- Ramond M‐J, Poynard T, Rueff B, Carey WD. Steroids in alcoholic hepatitis: another salvo of data. American Journal of Gastroenterology 1992;87(9):1219‐20. [CRS: 6800131000045543; EMBASE: 1992277170; CN‐00196579] - PubMed
-
- Ramond MJ, Poynard T, Rueff B, Horwitz RJ. Prednisolone for severe alcoholic hepatitis. Annals of Internal Medicine 1992;117(Suppl 2):36. [CRS: 6800131000045702; EMBASE: 1992360747]
-
- Ramond MJ, Poynard T, Rueff B, Mathurin P, Theodore C, Chaput JC, et al. A randomized trial of prednisolone in patients with severe alcoholic hepatitis. New England Journal of Medicine 1992;326(8):507‐12. [MEDLINE: ] - PubMed
Richardet 1993 {published data only}
-
- Mal F, Pham Huu T, Richardet JP, Roulot D, Labadie H, Pol S, et al. Corticosteroids (Cs) does not influence plasma tumor necrosis factor‐alpha (pTNF) and interleukin 6 (pIL6) concentrations in severe alcoholic hepatitis (AH): a cross over study. Hepatology (Baltimore, Md.) 1992;16:234A.
-
- Richardet JP, Dehoux M, Mal F, Roulot D, Labadie H, Pol S, et al. Influence of corticosteroids (CS) on plasma cytokines concentrations in patients with severe alcoholic hepatitis (HA): results of a randomized study. Journal of Hepatology 1993;18:S75.
Shumaker 1978 {published data only}
-
- Conn HO. Steroid treatment of alcoholic hepatitis. The yeas and the nays. Gastroenterology 1978;74(2 (Pt 1)):319‐22. [PUBMED: 620902] - PubMed
-
- Galambos JT. Alcoholic liver disease, new aspects of an old problem. Schweizerische Medizinische Wochenschrift 1978;108(28):1050‐2. [CENTRAL: 221034; CRS: 6800100000004689; EMBASE: 0978366839; JC‐‐NLM: Journal ID:uei, 0404401; PUBMED: 78228296] - PubMed
-
- Shumaker JB, Resnick RH, Galambos JT, Makopour H, Iber FL. A controlled trial of 6‐methylprednisolone in acute alcoholic hepatitis. With a note on published results in encephalopathic patients. American Journal of Gastroenterology 1978;69(4):443‐9. [MEDLINE: ] - PubMed
Theodossi 1982 {published data only}
Thursz 2015 {published data only}
-
- Petts G, Lloyd K, Vergis N, Kudo H, Quaglia A, Forrest E, et al. The liver biopsy in alcoholic hepatitis: data from the STeroids Or Pentoxifylline in Alcoholic Hepatitis (STOPAH) clinical trial. Gut 2015;64(Suppl 1):A262. [CRS: 6800131000059987; EMBASE: 72009796]
-
- Petts G, Lloyd K, Vergis N, Kudo H, Quaglia A, Forrest E, et al. The liver biopsy in alcoholic hepatitis: data from the STeroids Or Pentoxifylline in Alcoholic Hepatitis (STOPAH) clinical trial. Journal of Pathology 2015;237(Suppl 1):S23. [CRS: 6800131000075013; EMBASE: 72082064]
-
- Petts G, Lloyd K, Vergis N, Kudo H, Quaglia A, Forrest E, et al. Utility of liver biopsy in alcoholic hepatitis: data from the STeroids Or Pentoxyfilline in Alcoholic Hepatitis (STOPAH) clinical trial. Journal of Hepatology 2015;62(Suppl 2):S776‐7. [CRS: 6800131000059992; EMBASE: 71937847]
-
- Thursz M, Forrest E, Roderick P, Day C, Austin A, O'Grady J, et al. The clinical effectiveness and cost‐effectiveness of STeroids Or Pentoxifylline for Alcoholic Hepatitis (STOPAH): a 2 x 2 factorial randomised controlled trial. Health Technology Assessment 2015;19(102):1‐138. [CRS: 6800131000075049] - PMC - PubMed
References to studies excluded from this review
Alvarez 2004 {published data only}
-
- Alvarez MA, Cabre E, Lorenzo‐Zuniga V, Montoliu S, Planas R, Gassull MA. Combining steroids with enteral nutrition: a better therapeutic strategy for severe alcoholic hepatitis? Results of a pilot study. European Journal of Gastroenterology & Hepatology 2004;16:1375‐80. - PubMed
Cabré 2000 {published data only}
-
- Cabré E, Rodríguez‐Iglesias P, Caballería J, Quer JC, Sánchez‐Lombraña JL, Parés A, et al. Short‐ and long‐term outcome of severe alcohol‐induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology (Baltimore, Md.) 2000;32:36‐42. - PubMed
-
- Cabré E, on behalf of the Spanish Group for the Study of Alcoholic Hepatitis. Treatment of severe alcoholic hepatitis with steroids or total enteral nutrition: interim results of a prospective, randomized, multicentric trial. Gastroenterology 1998;114:A868‐9.
-
- Gassull M, Cabré E. Short and long‐term outcome in severe alcoholic hepatitis (SAH) treated with steroids or total enteral nutrition (TEN). A multicentric randomized controlled trial by the Spanish Group for the Study of Alcoholic Hepatitis. Journal of Parenteral and Enteral Nutrition 2000;24:S5.
Christensen 1981 {published data only}
-
- Christensen E, Fauerholdt L, Schlichting P, Juhl E, Poulsen H, Tygstrup N. Aspects of the natural history of gastrointestinal bleeding in cirrhosis and the effect of prednisone. Gastroenterology 1981;81(5):944‐52. - PubMed
Copenhagen 1969 {published data only}
-
- Copenhagen Study Group for Liver Diseases. Effect of prednisone on the survival of patients with cirrhosis of the liver. A report from the Copenhagen Study Group for Liver Diseases. Lancet 1969;1(586):119‐21. [CENTRAL: 844264; CRS: 6800100000024872; PUBMED: 69076529] - PubMed
Daures 1991 {published data only}
-
- Daures J‐P, Peray P, Bories P, Blanc P, Yousfi A, Michel H, et al. Steroid therapy in acute alcoholic hepatitis: a pooled estimate based on published randomized controlled trials [Place de la corticotherapie dans le traitement des hépatites alcooliques aiguës. Résultants d'une méta‐analyse]. Gastroenterologie Clinique et Biologique 1991;51:223‐8. - PubMed
Dhanda 2016 {published data only}
-
- Dhanda A, Collins P. Infection is common in patients with severe alcoholic hepatitis treated with steroids but not associated with poor outcome. Journal of Hepatology 2016;64:S228.
Galambos 1984 {published data only}
-
- Galambos JT, Riepe SP. Use of colchicine and steroids in the treatment of alcoholic liver disease. Recent Developments in Alcoholism 1984;2:181‐94. [CENTRAL: 34655; CRS: 6800100000000618; EMBASE: 6374780; JC‐‐NLM: Journal ID:rda, 8301996; PUBMED: 84222884] - PubMed
Gill 1984 {published data only}
-
- Gill R, Zieve L, Logan G. Severe alcoholic hepatitis improved by combined treatment with prednisolone, testosterone and an amino acid supplement. Hepatology (Baltimore, Md.) 1984;4:2894.
Goldis 2000 {published data only}
-
- Goldis A, Matei R, Vernic C, Strain R. Treatment of acute alcoholic hepatitis with glucocorticosteroids‐prognostic factors. Steatohepatitis (NASH and ASH). Falk Symposium 121 2000;123:25.
Hozo 1996 {published data only}
-
- Hozo I, Mise S, Rumboldt Z, Bagatin J, Tonkic A. A controlled clinical trial of methylprednisolone in patients with the cholestatic form of alcoholic liver cirrhosis [Kontrolirano klinicko ispitivanje metilprednisolona u bolesnika sa kolestatskim oblikom alkoholne ciroze jetre]. Medicinski Arhiv 1996;50:81‐3. [PMID: 9601759] - PubMed
-
- Hozo I, Mise S, Rumboldt Z, Bagatin J, Tonkic A. Controlled clinical trial of methylprednisolone in patients with the cholestatic form of alcoholic liver cirrhosis. Gastroenterology International 1997;10:137‐9. - PubMed
Imperiale 1990 {published data only}
-
- Imperiale TF, McCullough AJ. Do corticosteroids reduce mortality from alcoholic hepatitis? A meta‐analysis of the randomized trials. Annals of Internal Medicine 1990;113(4):299‐307. - PubMed
Lee 2016 {published data only}
-
- Lee Y‐S, Kim HJ, Kim JH, Yoo YJ, Kim TS, Kang SH, et al. Systematic review: steroid, pentoxifylline or combined therapy for acute alcoholic hepatitis. Hepatology International 2016;10(Suppl 1):424.
Lesesne 1978 {published data only}
-
- Lesesne HR, Bozymski EM, Fallon HJ. Treatment of alcoholic hepatitis with encephalopathy. Comparison of prednisolone with caloric supplements. Gastroenterology 1978;74(2 Pt 1):169‐73. [MEDLINE: ] - PubMed
Mal 1991 {published data only}
-
- Mal F, Huu TR, Roulot D, Pol S, Labadie H, Ricardet JP, et al. In severe alcoholic hepatitis (AH) plasma tumor necrosis factor (pTNF) is inconstantly elevated and is not influenced by corticosteroid (Cs) therapy. Hepatology (Baltimore, Md.) 1991;14(4 (Pt 2)):232A. [CENTRAL: 221590; CRS: 6800100000005112; CN‐00221590]
Mathurin 2018 {published data only}
-
- Mathurin P, Dufour JF, Bzowej NH, Shiffman ML, Arterburn S, Nguyen T, et al. Selonsertib in combination with prednisolone for the treatment of severe alcoholic hepatitis: a phase 2 randomized controlled trial. Hepatology 2018;68(1):8A‐9A.
Mendenhall 1993 {published data only}
-
- Mendenhall CL. Alcoholic hepatitis. In: Schiff L, Schiff ER editor(s). Diseases of the Liver. 7th Edition. Vol. 2, Philadelphia (PA): JB Lippincott Co, 1993:856‐74.
Moreno 2014 {published data only}
-
- Moreno C, Deltenre P, Senterre C, Louvet A, Gustot T, Bastens B, et al. Intensive enteral nutrition is ineffective for patients with severe alcoholic hepatitis treated with corticosteroids. Gastroenterology 2016;150:903‐10. - PubMed
-
- Moreno C, Trepo E, Louvet A, Degre D, Bastens B, Hittelet A, et al. Impact of intensive enteral nutrition in association with corticosteroids in the treatment of severe alcoholic hepatitis: a multicenter randomized controlled trial. Hepatology (Baltimore, Md.) 2014;60(Suppl 1):269A‐70A. [CRS: 6800131000024411; EMBASE: 71638823; CN‐01023754]
Morris 2005 {published data only}
-
- Morris JM, Forrest EH. Bilirubin response to corticosteroids in severe alcoholic hepatitis. European Journal of Gastroenterology & Hepatology 2005;17:759‐62. [PMID: 15947554] - PubMed
Naganuma 2014 {published data only}
-
- Naganuma A, Hoshino T, Ogashiwa T, Hayashi E, Uehara S, Miyamae N, et al. Pilot study of granulocytapheresis and leukocytapheresis for the treatment of severe alcoholic hepatitis. Hepatology International 2014;8(1 Suppl 1):S8. [CRS: 6800131000045556; CN‐01011068]
Naveau 2004 {published data only}
-
- Naveau S, Chollet‐Martin S, Dharancy S, Mathurin P, Jouet P, Piquet MA, et al. A double‐blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology (Baltimore, Md.) 2004;39:1390‐7. [PMID: 15122768] - PubMed
Phillips 2001 {published data only}
-
- Philips M, Curtis H, Portmann B, Donaldson N, Bomford A, O'Grady J. Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis: a randomized trial. Hepatology (Baltimore, Md.) 2001;34:250A. - PubMed
Poynard 1991 {published data only}
-
- Poynard T, Ramond MJ, Rueff B, Mathurin P, Chaput JC, Benhamou JP. Corticosteroids reduce mortality from alcoholic hepatitis in patients without encephalopathy. A meta‐analysis of the randomized trials (RCTs) including French trials. Hepatology (Baltimore, Md.) 1991;14:234A.
Reynolds 1989 {published data only}
-
- Reynolds TB, Benhamou JP, Blake J, Naccarato R, Orrego H. Treatment of acute alcoholic hepatitis. Gastroenterology International 1989;2(4):208‐16.
Schlichting 1976 {published data only}
-
- Schlichting P, Juhl E, Poulsen H, Winkel P. Alcoholic hepatitis superimposed on cirrhosis. Clinical significance and effect of long‐term prednisone treatment. Scandinavian Journal of Gastroenterology 1976;11:305‐12. [MEDLINE: ] - PubMed
Singal 2018 {published data only}
Spahr 2002 {published data only}
-
- Spahr L, Rubbia‐Brandt L, Giostra E, Rougemont A‐L, Pugin J, Fischer M, et al. Combination of steroids with anti‐TNFa (INFLIXIMAB) or placebo in severe alcoholic hepatitis: early changes in ICAM‐1, histology and biochemical parameters. A pilot study. Journal of Hepatology 2002;37:448‐55. [PMID: 12217597] - PubMed
Stewart 2002 {published data only}
-
- Stewart S, Prince M, Bassendine M, Hudson M, James O, Jones D, et al. A trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. Journal of Hepatology 2002;36(Suppl 1):42. - PubMed
Szabo 2018 {published data only}
-
- Szabo G, Mitchell MC, McClain CJ, Dasarathy S, McCullough AJ, Nagy L, et al. IL‐1 receptor antagonist in combination with pentoxifylline and zinc for severe alcoholic hepatitis: a multicenter randomized double‐bind placebo‐controlled clinical trial. Hepatology 2018;68(1):1445A.
Tygstrup 1979 {published data only}
-
- Tygstrup N, Christensen E, Juhl E. Randomised clinical trials in hepatology [Randomisierte klinische therapiestudien in der hepatologie]. Internist 1979;20:565‐70. - PubMed
References to ongoing studies
NCT03160651 {published data only}
-
- Corticosteroids in severe alcoholic hepatitis patients with early spontaneous improvement. Ongoing study Estimated study start date: June 2017.
Additional references
AASLD 2010
-
- O'Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology (Baltimore, Md.) 2010;51:307‐28. - PubMed
Balshem 2011
-
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] - PubMed
Becker 1996
-
- Becker U, Deis A, Sørensen TI, Grønbaek M, Borch‐Johnsen K, Müller CF, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology (Baltimore, Md.) 1996;23:1025‐9. - PubMed
Beckett 1961
Begg 1994
-
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [PUBMED: 7786990] - PubMed
Brok 2008
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial Sequential Analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. - PubMed
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive – Trial Sequential Analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. - PubMed
Buzzetti 2017
Castellini 2017
-
- Castellini G, Nielsen E, Gluud, C. Comment on: "Cell therapy for heart disease: Trial Sequential Analyses of two Cochrane Reviews". Clinical Pharmacology and Therapeutics 2017;102:21‐4. - PubMed
Christensen 1995
Christensen 1999
-
- Christensen E, Gluud C. Glucocorticosteroids are not effective in alcoholic hepatitis. American Journal of Gastroenterology 1999;94(10):3065‐6. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
DeMets 1987
-
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Dominguez 2008
-
- Dominguez M, Rincon D, Abraldes JG, Miquel R, Colmenero J, Bellot P, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. American Journal of Gastroenterology 2008;103:2747‐56. - PubMed
Dunn 2005
-
- Dunn W, Jamil LH, Brown LS, Wiesner RH, Kim WR, Menon KV, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology (Baltimore, Md.) 2005;41:353‐8. - PubMed
EASL 2012
-
- European Association for the Study of the Liver. EASL Clinical Practical Guidelines: management of alcoholic liver disease. Journal of Hepatology 2012;57:399‐420. - PubMed
EASL 2018
-
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of alcohol‐related liver disease. Journal of Hepatology 2018;69:154‐81. - PubMed
Egger 1997
Ellis 2012
-
- Ellis EL, Mann DA. Clinical evidence for the regression of liver fibrosis. Journal of Hepatology 2012;56(5):1171‐80. - PubMed
Feinberg 2017
Fisher 1922
-
- Fisher RA. On the interpretation of X^2 from contingency tables, and the calculation of P. Journal of the Royal Statistical Society 1922;85(1):87‐94.
Forrest 2005
French 1912
-
- French H. An index of differential diagnosis of main symptoms. Wright.Bristol 1912:368.
Garattini 2016
-
- Garattini S, Jakobsen JC, Wetterslev J, Bertelé V, Banzi R, Rath A, et al. Evidence‐based clinical practice: overview of threats to the validity of evidence and how to minimise them. European Journal of Internal Medicine 2016;32:13‐21. - PubMed
Gerber 1973
-
- Gerber MA, Orr W, Denk H, Schaffner F, Popper H. Hepatocellular hyalin in cholestasis and cirrhosis: its diagnostic significance. Gastroenterology 1973;64(1):89‐98. - PubMed
Gluud 2017
-
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato‐Biliary Group. About Cochrane (Cochrane Review Groups (CRGs)) 2017, Issue 2. Art. No.: LIVER 2017.
GRADEpro GDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2011a
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] - PubMed
Guyatt 2011b
-
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [PUBMED: 21194891] - PubMed
Guyatt 2011c
-
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence – study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] - PubMed
Guyatt 2011d
-
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence – publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] - PubMed
Guyatt 2011e
-
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence – imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] - PubMed
Guyatt 2011f
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence – inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] - PubMed
Guyatt 2011g
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence – indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] - PubMed
Guyatt 2011h
-
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] - PubMed
Guyatt 2013a
-
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151‐7. [PUBMED: 22542023] - PubMed
Guyatt 2013b
-
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables – binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158‐72. [PUBMED: 22609141] - PubMed
Guyatt 2013c
-
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles – continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173‐83. [PUBMED: 23116689] - PubMed
Guyatt 2017
-
- Guyatt GH, Ebrahim S, Alonso‐Coello P, Johnston BC, Mathioudakis AG, Briel M, et al. GRADE guidelines 17: assessing the risk of bias associated with missing participant outcome data in a body of evidence. Journal of Clinical Epidemiology 2017;87:14‐22. [PUBMED: 28529188] - PubMed
Harbord 2006
-
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. - PubMed
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2011a
-
- Higgins JP, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Houghton 2009
-
- Houghton M. Discovery of the hepatitis C virus. Liver International 2009;29(Suppl 1):82‐8. - PubMed
ICH‐GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, Philadelphia (PA): Barnett International/PAREXEL, 1997.
Ioannidis 2009
-
- Ioannidis JP. Adverse events in randomized trials: neglected, restricted, distorted, and silenced. Archives of Internal Medicine 2009;169(19):1737‐9. - PubMed
Jakobsen 2014
Jensen 1994
-
- Jensen K, Gluud C. The Mallory body: morphological, clinical and experimental studies (Part 1 of a literature survey). Hepatology (Baltimore, Md.) 1994;20:1061‐77. [MEDLINE: ] - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Lefkowitch 2005
-
- Lefkowitch JH. Morphology of alcoholic liver disease. Clinics in Liver Disease 2005;9(1):37‐53. - PubMed
Lieber 1999
-
- Lieber CS. Role of S‐adenosyl‐L‐methionine in the treatment of liver diseases. Journal of Hepatology 1999;30:1155‐9. - PubMed
Louvet 2007
-
- Louvet A, Naveau S, Abdelnour M, Ramond MJ, Diaz E, Fartoux L. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology (Baltimore, Md.) 2007;45(6):1348‐54. - PubMed
Louvet 2018
-
- Louvet A, Thursz M, Kim DJ, Labreuche J, Atkinson SR, Sidhu SS, et al. Corticosteroids reduce risk of death within 28 days for patients with severe alcoholic hepatitis, compared with pentoxifylline or placebo – a meta‐analysis of individual data from controlled trials. Gastroenterology 2018;155:458‐68. - PubMed
Lundh 2017
Mathurin 2011
-
- Mathurin P, O'Grady J, Carithers RL, Phillips M, Louvet A, Mendenhall CL, et al. Corticosteroids improve short‐term survival in patients with severe alcoholic hepatitis: meta‐analysis of individual patient data. Gut 2011;60(2):255‐60. - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. - PubMed
Mustafa 2013
-
- Mustafa RA, Santesso N, Brozek J, Akl EA, Walter SD, Norman G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. Journal of Clinical Epidemiology 2013;66(7):736‐42; quiz 742.e1‐5. [PUBMED: 23623694] - PubMed
NTAWG 2015
-
- Nordic Trial Alliance Working Group on Transparency and Registration. Transparency and registration in clinical research in the Nordic countries (Report), 2015. nta.nordforsk.org/projects/nta_transparency_report.pdf (accessed 21 June 2017).
Petrasek 2013
-
- Petrasek J, Iracheta‐Vellve A, Csak T, Satishchandran A, Kodys K, Kurt‐Jones EA, et al. STING‐IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proceedings of the National Academy of Sciences of the United States of America 2013;110(41):16544‐9. - PMC - PubMed
Phillips 2006
-
- Phillips M, Curtis H, Portmann B, Donaldson N, Bomford A, O'Grady J. Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis – a randomised clinical trial. Journal of Hepatology 2006;44:784‐90. - PubMed
Pugh 1973
-
- Pugh RN, Murray‐Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. British Journal of Surgery 1973;60:646‐9. - PubMed
Rambaldi 2006
Rambaldi 2008
-
- Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C. Systematic review: glucocorticosteroids for alcoholic hepatitis – a Cochrane Hepato‐Biliary Group systematic review with meta‐analyses and trial sequential analyses of randomized clinical trials. Alimentary Pharmacology & Therapeutics 2008;27(12):1167‐78. [CRS: 6800131000005200; EMBASE: 2008243224; JC‐‐NLM: Journal ID:a5d, 8707234; PUBMED: 18363896] - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rhen 2005
-
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids – new mechanisms for old drugs. New England Journal of Medicine 2005;353(16):1711‐23. - PubMed
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane Reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Rücker 2008
-
- Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta‐analyses with binary outcomes. Statistics in Medicine 2008;27:746‐63. - PubMed
Savović 2012a
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. - PubMed
Savović 2012b
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes, R, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment in controlled trials. JAMA 1995;273:408‐12. [MEDLINE: ] - PubMed
Schäcke 2002
-
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacology & Therapeutics 2002;96(1):23‐43. - PubMed
Spahr 2001
-
- Spahr L, Rubbia‐Brandt L, Pugin J, Giostra E, Frossard JL, Borisch B, et al. Rapid changes in alcoholic hepatitis histology under steroids: correlation with soluble intercellular adhesion molecule‐1 in hepatic venous blood. Journal of Hepatology 2001;35(5):582‐9. - PubMed
Sterne 2011
-
- Sterne JA, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Student 1908
-
- Student. The probable error of a mean. Biometrika 1908;6(1):1‐25.
Taïeb 2000
-
- Taïeb J, Mathurin P, Elbim C, Cluzel P, Arce‐Vicioso M, Bernard B, et al. Blood neutrophil functions and cytokine release in severe alcoholic hepatitis: effect of corticosteroids. Journal of Hepatology 2000;32(4):579‐86. - PubMed
Thorlund 2009
-
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. - PubMed
Thorlund 2010
Thorlund 2011
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA), 2011. ctu.dk/tsa/files/tsa_manual.pdf (accessed 21 June 2017).
TSA 2011 [Computer program]
-
- Copenhagen Trial Unit. TSA – Trial Sequential Analysis. Version 0.9 Beta. Copenhagen: Copenhagen Trial Unit, 2011.
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial Sequential Analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. - PubMed
Wetterslev 2009
Wetterslev 2017
WHO 2010
-
- World Health Organization. Global strategy to reduce the harmful use of alcohol, 2010. www.who.int/substance_abuse/msbalcstragegy.pdf (accessed 21 June 2017).
WHO 2013
-
- Sauced RS. Update on background paper 6.14. Harmful use of alcohol, alcohol use, disorders, and alcoholic liver diseases, 2013. www.who.int/medicines/areas/priority_medicines/BP6_14Alcohol.pdf (accessed 21 June 2017).
Wood 2008
Wu 1999
-
- Wu D, Cederbaum AI. Ethanol‐induced apoptosis to stable HepG2 cell lines expressing human cytochrome P‐4502E1. Alcoholism: Clinical and Experimental Research 1999;23:67‐76. - PubMed
References to other published versions of this review
Pavlov 2016
Pavlov 2017
Saconato 1999
-
- Saconato H, Gluud C, Christensen E, Atallah ÁN. Glucocorticosteroids for alcoholic hepatitis. Cochrane Database of Systematic Reviews 1999, Issue 1. [DOI: 10.1002/14651858.CD001511] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

