Palmitoylation by Multiple DHHC Enzymes Enhances Dopamine Transporter Function and Stability
- PMID: 30965003
- PMCID: PMC6746250
- DOI: 10.1021/acschemneuro.8b00558
Palmitoylation by Multiple DHHC Enzymes Enhances Dopamine Transporter Function and Stability
Abstract
The dopamine transporter (DAT) is a plasma membrane protein that mediates the reuptake of extracellular dopamine (DA) and controls the spatiotemporal dynamics of dopaminergic neurotransmission. The transporter is subject to fine control that tailors clearance of transmitter to physiological demands, and dysregulation of reuptake induced by psychostimulant drugs, transporter polymorphisms, and signaling defects may impact transmitter tone in disease states. We previously demonstrated that DAT undergoes complex regulation by palmitoylation, with acute inhibition of the modification leading to rapid reduction of transport activity and sustained inhibition of the modification leading to transporter degradation and reduced expression. Here, to examine mechanisms and outcomes related to increased modification, we coexpressed DAT with palmitoyl acyltransferases (PATs), also known as DHHC enzymes, which catalyze palmitate addition to proteins. Of 12 PATs tested, DAT palmitoylation was stimulated by DHHC2, DHHC3, DHHC8, DHHC15, and DHHC17, with others having no effect. Increased modification was localized to previously identified palmitoylation site Cys580 and resulted in upregulation of transport kinetics and elevated transporter expression mediated by reduced degradation. These findings confirm palmitoylation as a regulator of multiple DAT properties crucial for appropriate DA homeostasis and identify several potential PAT pathways linked to these effects. Defects in palmitoylation processes thus represent possible mechanisms of transport imbalances in DA disorders.
Keywords: [S]-methionine labeling; acyl protein thioesterase; palmitoyl acyl transferase; post-translational modification; protein degradation; protein palmitoyl thioesterase.
Conflict of interest statement
Figures

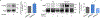
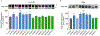
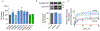
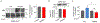
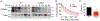
References
-
- Torres GE, Gainetdinov RR, and Caron MG (2003) Plasma membrane monoamine transporters: structure, regulation and function, Nat Rev Neurosci 4, 13–25. - PubMed
-
- Iversen SD, and Iversen LL (2007) Dopamine: 50 years in perspective, Trends Neurosci 30, 188–193. - PubMed
-
- Eriksen J, Jorgensen TN, and Gether U (2010) Regulation of dopamine transporter function by protein-protein interactions: new discoveries and methodological challenges, J Neurochem 113, 27–41. - PubMed
-
- Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Loland CJ, Stromgaard K, and Gether U (2011) SLC6 neurotransmitter transporters: structure, function, and regulation, Pharmacol Rev 63, 585–640. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

