Open-Label Clinical Trials of Oral Pulse Dexamethasone for Adults with Idiopathic Nephrotic Syndrome
- PMID: 30965344
- PMCID: PMC6677391
- DOI: 10.1159/000497064
Open-Label Clinical Trials of Oral Pulse Dexamethasone for Adults with Idiopathic Nephrotic Syndrome
Abstract
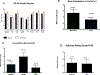
Background: In adults with primary focal segmental glomerulosclerosis (FSGS), daily prednisone may induce complete remissions (CR) and partial remissions (PR), but relapses are frequent and adverse events are common.
Methods: We carried out 2 open-label, uncontrolled trials to explore the efficacy and tolerability of pulse oral dexamethasone as an alternative to daily prednisone. We enrolled adult patients with proteinuria > 3.5 g/day despite the use of renin-angiotensin-aldosterone blockade. In the first trial, we enrolled 14 subjects with FSGS and administered 4 dexamethasone doses (25 mg/m2) daily for 4 days, repeated every 28 days over 32 weeks. The second trial involved a more intensive regimen. Eight subjects received 4 dexamethasone doses of 50 mg/m2 every 4 weeks for 12 weeks, followed by 4 doses of 25 mg/m2 every 4 weeks for 36 weeks; subjects were randomized to 2 doses every 2 weeks or 4 doses every 4 weeks.
Results: In the first trial, we enrolled 13 subjects with FSGS and 1 with minimal change disease and found a combined CR and PR rate of 36%. In the second trial, we enrolled 8 subjects. The combined CR and PR rate was 29%. Analysis combining both trials showed a combined CR and PR rate of 33%. Adverse events were observed in 32% of subjects, with mood symptoms being most common. There were no serious adverse events related to the study.
Conclusion: We conclude that high dose oral dexamethasone is well tolerated by adults with idiopathic nephrotic syndrome and may have some efficacy.
Keywords: Glucocorticoids; Proteinuria; Remission; Steroids.
Published by S. Karger AG, Basel.
Figures

References
-
- Deegens JK, Steenbergen EJ, Wetzels JF. Review on diagnosis and treatment of focal segmental glomerulosclerosis. Neth JMed. 2008;66(1): 3–12. - PubMed
-
- Ponticelli C, Villa M, Banfi G, et al. Can prolonged treatment improve the prognosis in adults with focal segmental glomerulosclerosis? American Journal of Kidney Diseases. 1999;34(4): 618–625. - PubMed
-
- Boros P, Ochando J, Zeher M. Myeloid derived suppressor cells and autoimmunity. Hum Immunol. 2016;77(8): 631–636. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

