Soritesidine, a Novel Proteinous Toxin from the Okinawan Marine Sponge Spongosorites sp
- PMID: 30965587
- PMCID: PMC6520796
- DOI: 10.3390/md17040216
Soritesidine, a Novel Proteinous Toxin from the Okinawan Marine Sponge Spongosorites sp
Abstract
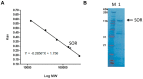
A novel protein, soritesidine (SOR) with potent toxicity was isolated from the marine sponge Spongosorites sp. SOR exhibited wide range of toxicities over various organisms and cells including brine shrimp (Artemia salina) larvae, sea hare (Aplysia kurodai) eggs, mice, and cultured mammalian cells. Toxicities of SOR were extraordinary potent. It killed mice at 5 ng/mouse after intracerebroventricular (i.c.v.) injection, and brine shrimp and at 0.34 µg/mL. Cytotoxicity for cultured mammalian cancer cell lines against HeLa and L1210 cells were determined to be 0.062 and 12.11 ng/mL, respectively. The SOR-containing fraction cleaved plasmid DNA in a metal ion dependent manner showing genotoxicity of SOR. Purified SOR exhibited molecular weight of 108.7 kDa in MALDI-TOF MS data and isoelectric point of approximately 4.5. N-terminal amino acid sequence up to the 25th residue was determined by Edman degradation. Internal amino acid sequences for fifteen peptides isolated from the enzyme digest of SOR were also determined. None of those amino acid sequences showed similarity to existing proteins, suggesting that SOR is a new proteinous toxin.
Keywords: brine shrimp; cytotoxicity; genotoxin; mouse lethality; protein; sponge.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Cyanobacterial cytotoxicity versus toxicity to brine shrimp Artemia salina.Toxicon. 2011 Jan;57(1):76-83. doi: 10.1016/j.toxicon.2010.10.002. Epub 2010 Oct 12. Toxicon. 2011. PMID: 20946912
-
A comparison between two brine shrimp assays to detect in vitro cytotoxicity in marine natural products.BMC Biotechnol. 2002 Sep 23;2:17. doi: 10.1186/1472-6750-2-17. BMC Biotechnol. 2002. PMID: 12270067 Free PMC article.
-
Novel proteinaceous toxins from the nematocyst venom of the Okinawan sea anemone Phyllodiscus semoni Kwietniewski.Biochem Biophys Res Commun. 2002 Jun 21;294(4):760-3. doi: 10.1016/S0006-291X(02)00547-8. Biochem Biophys Res Commun. 2002. PMID: 12061771
-
Altohyrtins B and C and 5-desacetylaltohyrtin A, potent cytotoxic macrolide congeners of altohyrtin A, from the Okinawan marine sponge Hyrtios altum.Chem Pharm Bull (Tokyo). 1993 May;41(5):989-91. doi: 10.1248/cpb.41.989. Chem Pharm Bull (Tokyo). 1993. PMID: 8339346
-
Marine spongean cytotoxins.J Nat Toxins. 1999 Jun;8(2):249-58. J Nat Toxins. 1999. PMID: 10410335 Review.
Cited by
-
Marine natural products as a source of novel anticancer drugs: an updated review (2019-2023).Nat Prod Bioprospect. 2025 Jan 24;15(1):13. doi: 10.1007/s13659-024-00493-5. Nat Prod Bioprospect. 2025. PMID: 39853457 Free PMC article. Review.
References
-
- Fusetani N., Matsunaga S. Bioactive sponge peptides. Chem. Rev. 1993;93:1793–1806. doi: 10.1021/cr00021a007. - DOI
-
- Li H., Bowling J.J., Fronczek F.R., Hong J., Jabba S.V., Murray T.F., Ha N.-C., Hamann M.T., Jung J.H. Asteropsin a: An unusual cystine-crosslinked peptide from porifera enhances neuronal Ca2+ influx. Biochim. Biophys. Acta. 2013;1830:2591–2599. doi: 10.1016/j.bbagen.2012.11.015. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

