Neuroprotection, Recovery of Function and Endogenous Neurogenesis in Traumatic Spinal Cord Injury Following Transplantation of Activated Adipose Tissue
- PMID: 30965679
- PMCID: PMC6523261
- DOI: 10.3390/cells8040329
Neuroprotection, Recovery of Function and Endogenous Neurogenesis in Traumatic Spinal Cord Injury Following Transplantation of Activated Adipose Tissue
Abstract
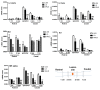
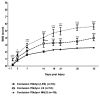
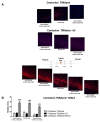
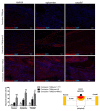
Spinal cord injury (SCI) is a devastating disease, which leads to paralysis and is associated to substantially high costs for the individual and society. At present, no effective therapies are available. Here, the use of mechanically-activated lipoaspirate adipose tissue (MALS) in a murine experimental model of SCI is presented. Our results show that, following acute intraspinal MALS transplantation, there is an engraftment at injury site with the acute powerful inhibition of the posttraumatic inflammatory response, followed by a significant progressive improvement in recovery of function. This is accompanied by spinal cord tissue preservation at the lesion site with the promotion of endogenous neurogenesis as indicated by the significant increase of Nestin-positive cells in perilesional areas. Cells originated from MALS infiltrate profoundly the recipient cord, while the extra-dural fat transplant is gradually impoverished in stromal cells. Altogether, these novel results suggest the potential of MALS application in the promotion of recovery in SCI.
Keywords: adipose tissue; cell therapies; inflammation; mechanical activation; neurogenesis; neuroprotection; spinal cord injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- White B.A.B., Dea N., Street J.T., Cheng C.L., Rivers C.S., Attabib N., Kwon B.K., Fisher C.G., Dvorak M.F. The Economic Burden of Urinary Tract Infection and Pressure Ulceration in Acute Traumatic Spinal Cord Injury Admissions: Evidence for Comparative Economics and Decision Analytics from a Matched Case-Control Study. J. Neurotrauma. 2017;34:2892–2900. doi: 10.1089/neu.2016.4934. - DOI - PubMed

