Polyol Structure and Ionic Moieties Influence the Hydrolytic Stability and Enzymatic Hydrolysis of Bio-Based 2,5-Furandicarboxylic Acid (FDCA) Copolyesters
- PMID: 30965704
- PMCID: PMC6418894
- DOI: 10.3390/polym9090403
Polyol Structure and Ionic Moieties Influence the Hydrolytic Stability and Enzymatic Hydrolysis of Bio-Based 2,5-Furandicarboxylic Acid (FDCA) Copolyesters
Abstract
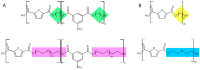
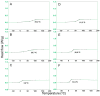
A series of copolyesters based on furanic acid and sulfonated isophthalic acid with various polyols were synthetized and their susceptibility to enzymatic hydrolysis by cutinase 1 from Thermobifida cellulosilytica (Thc_Cut1) investigated. All copolyesters consisted of 30 mol % 5-sulfoisophthalate units (NaSIP) and 70 mol % 2,5-furandicarboxylic acid (FDCA), while the polyol component was varied, including 1,2-ethanediol, 1,4-butanediol, 1,8-octanediol, diethylene glycol, triethylene glycol, or tetraethylene glycol. The composition of the copolyesters was confirmed by ¹H-NMR and the number average molecular weight (Mn) was determined by GPC to range from 2630 to 8030 g/mol. A DSC analysis revealed glass-transition temperatures (Tg) from 84 to 6 °C, which were decreasing with increasing diol chain length. The crystallinity was below 1% for all polyesters. The hydrolytic stability increased with the chain length of the alkyl diol unit, while it was generally higher for the ether diol units. Thc_Cut1 was able to hydrolyze all of the copolyesters containing alkyl diols ranging from two to eight carbon chain lengths, while the highest activities were detected for the shorter chain lengths with an amount of 13.6 ± 0.7 mM FDCA released after 72 h of incubation at 50 °C. Faster hydrolysis was observed when replacing an alkyl diol by ether diols, as indicated, e.g., by a fivefold higher release of FDCA for triethylene glycol when compared to 1,8-octanediol. A positive influence of introducing ionic phthalic acid was observed while the enzyme preferentially cleaved ester bonds associated to the non-charged building blocks.
Keywords: Thermobifida cellulosilytica; bio-based; cutinase; poly(2,5-furan dicarboxylate); sulfonated isophthalic acid.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures








References
-
- Tsanaktsis V., Papageorgiou G.Z., Bikiaris D.N. A facile method to synthesize high-molecular-weight biobased polyesters from 2,5-furandicarboxylic acid and long-chain diols. J. Polym. Sci. Part A Polym. Chem. 2015;53:2617–2632. doi: 10.1002/pola.27730. - DOI
-
- Papageorgiou G.Z., Tsanaktsis V., Papageorgiou D.G., Exarhopoulos S., Papageorgiou M., Bikiaris D.N. Evaluation of polyesters from renewable resources as alternatives to the current fossil-based polymers. Phase transitions of poly(butylene 2,5-furan-dicarboxylate) Polymer. 2014;55:3846–3858. doi: 10.1016/j.polymer.2014.06.025. - DOI
-
- Papageorgiou G.Z., Papageorgiou D.G., Terzopoulou Z., Bikiaris D.N. Production of bio-based 2,5-furan dicarboxylate polyesters: Recent progress and critical aspects in their synthesis and thermal properties. Eur. Polym. J. 2016;83:202–229. doi: 10.1016/j.eurpolymj.2016.08.004. - DOI
-
- Werpy T., Petersen G. Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas. National Renewable Energy Lab; Golden, CO, USA: 2004.
LinkOut - more resources
Full Text Sources
Miscellaneous

