Functional Aromatic Polyamides
- PMID: 30965723
- PMCID: PMC6419023
- DOI: 10.3390/polym9090414
Functional Aromatic Polyamides
Abstract
We describe herein the state of the art following the last 8 years of research into aromatic polyamides, wholly aromatic polyamides or aramids. These polymers belong to the family of high performance materials because of their exceptional thermal and mechanical behavior. Commercially, they have been transformed into fibers mainly for production of advanced composites, paper, and cut and fire protective garments. Huge research efforts have been carried out to take advantage of the mentioned characteristics in advanced fields related to transport applications, optically active materials, electroactive materials, smart materials, or materials with even better mechanical and thermal behavior.
Keywords: advanced materials; aramids; aromatic polyamides; functional polymers; high-performance polymers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









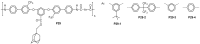
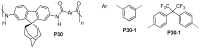


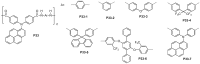



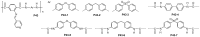





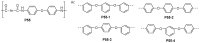
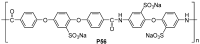




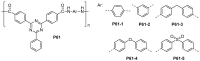
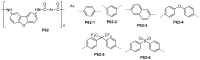


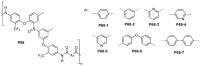

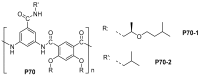


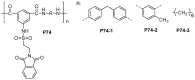

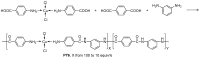
References
-
- Rules and Regulations under the Textile Fiber Products Identification Act in Title 16. Commercial Practices, Subchapter A, Chapter I, Part 303.7, Electronic Code of Federal Regulations (e-CFR) [(accessed on 29 May 2017)]; Available online: https://www.ecfr.gov/cgi-bin/text-idx?SID=d686e726b95f4e97353f2ee3103c76....
-
- García J.M., García F.C., Serna F., de la Peña J.L. High-performance aromatic polyamides. Prog. Polym. Sci. 2010;35:623–686. doi: 10.1016/j.progpolymsci.2009.09.002. - DOI
-
- Marchildon K. Polyamides—Still strong after seventy years. Macromol. React. Eng. 2011;5:22–54. doi: 10.1002/mren.201000017. - DOI
-
- Yamazaki N., Higasi F., Kawataba J. Studies on reactions of the N-phosphonium salts of pyridines. XI. Preparation of polypeptides and polyamides by means of triaryl phosphites in pyridine. J. Polym. Sci. Polym. Chem. 1974;12:2149–2154. doi: 10.1002/pol.1974.170120935. - DOI
-
- Trigo-López M., Estevez P., San-José N., Gomez-Valdemoro A., García F.C., Serna F., de la Peña J.L., Garcia J.M. Recent Patents on Aromatic Polyamides. Recent Pat. Mater. Sci. 2009;2:190–208. doi: 10.2174/1874464810902030190. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

