Synthesis and Characterization of Multifunctional Two-Component Waterborne Polyurethane Coatings: Fluorescence, Thermostability and Flame Retardancy
- PMID: 30965795
- PMCID: PMC6418666
- DOI: 10.3390/polym9100492
Synthesis and Characterization of Multifunctional Two-Component Waterborne Polyurethane Coatings: Fluorescence, Thermostability and Flame Retardancy
Abstract
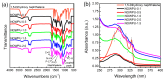
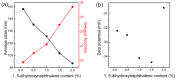
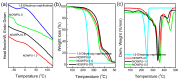
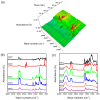
Fluorescent and flame-retardant two-component waterborne polyurethane coatings were synthesized using 1,5-dihydroxy naphthalene, a halogen-free polyphosphate and a hydrophilic curing agent, and their properties were systematically characterized. The average particle sizes and zeta potential values were below 170 nm and -30 mV. Meanwhile, the multifunctional two-component waterborne polyurethane coatings had strong fluorescence intensities. When comparing with the coatings with 0.5 wt % 1,5-dihydroxy naphthalene, the coatings with 1.0 wt % 1,5-dihydroxy naphthalene had a stronger microphase separation. Interestingly, the thermostability of the multifunctional coatings was remarkably improved through 1.0 wt % 1,5-dihydroxy naphthalene, and besides it belonged to nonflammable materials. Additionally, all of the coating films passed the solvent resistance testing. These samples with different amounts of 1,5-dihydroxy naphthalene are environmental friendly, especially applications that require transparent and fluorescent coatings.
Keywords: TGA-FTIR; coatings; flame retardancy; fluorescence; two-component waterborne polyurethane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Chung Y.C., Yang K., Choi J.W., Chun B.C. Characterisation and application of polyurethane copolymers grafted with photoluminescent dyes. Color. Technol. 2014;130:305–313. doi: 10.1111/cote.12097. - DOI
-
- Chung Y.C., Choi J.W., Lee H.L., Chun B.C. The characterization and effects of the alizarin series dyes grafted to polyurethane copolymers on the photoluminescence and low temperature flexibility. Fibers Polym. 2014;15:8–17. doi: 10.1007/s12221-014-0008-3. - DOI
-
- Guo L., Wang L., Huang S., Qu J. Synthesis and properties of novel water-dispersible polyisocyanates. J. Appl. Polym. Sci. 2017;134:44735. doi: 10.1002/app.44735. - DOI
-
- Ge Z., Luo Y. Synthesis and characterization of siloxane-modified two-component waterborne polyurethane. Prog. Org. Coat. 2013;76:1522–1526. doi: 10.1016/j.porgcoat.2013.06.007. - DOI
-
- Wu G., Li J., Chai C., Ge Z., Lin J., Luo Y. Synthesis and characterization of novel post-chain extension flame retardant waterborne polyurethane. RSC Adv. 2015;5:97710–97719. doi: 10.1039/C5RA12975C. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

