Immobilization of Platelet-Rich Plasma onto COOH Plasma-Coated PCL Nanofibers Boost Viability and Proliferation of Human Mesenchymal Stem Cells
- PMID: 30966035
- PMCID: PMC6418517
- DOI: 10.3390/polym9120736
Immobilization of Platelet-Rich Plasma onto COOH Plasma-Coated PCL Nanofibers Boost Viability and Proliferation of Human Mesenchymal Stem Cells
Abstract
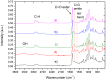
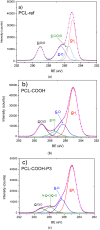
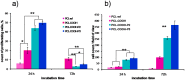
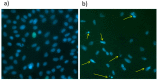
The scaffolds made of polycaprolactone (PCL) are actively employed in different areas of biology and medicine, especially in tissue engineering. However, the usage of unmodified PCL is significantly restricted by the hydrophobicity of its surface, due to the fact that its inert surface hinders the adhesion of cells and the cell interactions on PCL surface. In this work, the surface of PCL nanofibers is modified by Ar/CO₂/C₂H₄ plasma depositing active COOH groups in the amount of 0.57 at % that were later used for the immobilization of platelet-rich plasma (PRP). The modification of PCL nanofibers significantly enhances the viability and proliferation (by hundred times) of human mesenchymal stem cells, and decreases apoptotic cell death to a normal level. According to X-ray photoelectron spectroscopy (XPS), after immobilization of PRP, up to 10.7 at % of nitrogen was incorporated into the nanofibers surface confirming the grafting of proteins. Active proliferation and sustaining the cell viability on nanofibers with immobilized PRP led to an average number of cells of 258 ± 12.9 and 364 ± 34.5 for nanofibers with ionic and covalent bonding of PRP, respectively. Hence, our new method for the modification of PCL nanofibers with PRP opens new possibilities for its application in tissue engineering.
Keywords: COOH plasma; PRP immobilization; cell viability; nanofibers; platelet-rich plasma; polycaprolactone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Yao Q., Cosme J.G.L., Xu T., Miszuk J.M., Picciani P.H.S., Fong H., Sun H. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials. 2017;115:115–127. doi: 10.1016/j.biomaterials.2016.11.018. - DOI - PMC - PubMed
-
- Woodruff M.A., Hutmacher D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010;35:1217–1256. doi: 10.1016/j.progpolymsci.2010.04.002. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

