Metal Free Reversible-Deactivation Radical Polymerizations: Advances, Challenges, and Opportunities
- PMID: 30966069
- PMCID: PMC6415071
- DOI: 10.3390/polym10010035
Metal Free Reversible-Deactivation Radical Polymerizations: Advances, Challenges, and Opportunities
Abstract
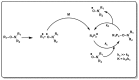
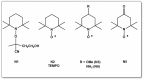
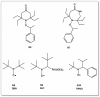
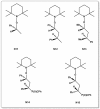
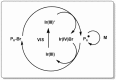
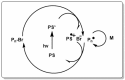
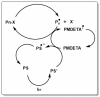
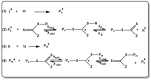
A considerable amount of the worldwide industrial production of synthetic polymers is currently based on radical polymerization methods. The steadily increasing demand on high performance plastics and tailored polymers which serve specialized applications is driven by the development of new techniques to enable control of polymerization reactions on a molecular level. Contrary to conventional radical polymerization, reversible-deactivation radical polymerization (RDRP) techniques provide the possibility to prepare polymers with well-defined structures and functionalities. The review provides a comprehensive summary over the development of the three most important RDRP methods, which are nitroxide mediated radical polymerization, atom transfer radical polymerization and reversible addition fragmentation chain transfer polymerization. The focus thereby is set on the newest developments in transition metal free systems, which allow using these techniques for biological or biomedical applications. After each section selected examples from materials synthesis and application to biomedical materials are summarized.
Keywords: ATRP; NMRP; RAFT; organic synthesis; polymer chemistry; reversible-deactivation radical polymerization.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Polyhomologation. A living C1 polymerization.Acc Chem Res. 2010 Nov 16;43(11):1420-33. doi: 10.1021/ar100062a. Epub 2010 Sep 8. Acc Chem Res. 2010. PMID: 20825177 Review.
-
Reversible Deactivation Radical Polymerization of Monomers Containing Activated Aziridine Groups.Macromol Rapid Commun. 2016 Oct;37(20):1694-1700. doi: 10.1002/marc.201600354. Epub 2016 Aug 22. Macromol Rapid Commun. 2016. PMID: 27548069
-
Toward Green Atom Transfer Radical Polymerization: Current Status and Future Challenges.Adv Sci (Weinh). 2022 Jul;9(19):e2106076. doi: 10.1002/advs.202106076. Epub 2022 Feb 17. Adv Sci (Weinh). 2022. PMID: 35175001 Free PMC article. Review.
-
Metal-Free Organocatalyzed Atom Transfer Radical Polymerization: Synthesis, Applications, and Future Perspectives.Macromol Rapid Commun. 2021 Aug;42(15):e2100221. doi: 10.1002/marc.202100221. Epub 2021 Jul 4. Macromol Rapid Commun. 2021. PMID: 34223686 Review.
-
Surface modification of electrospun fibres for biomedical applications: A focus on radical polymerization methods.Biomaterials. 2016 Nov;106:24-45. doi: 10.1016/j.biomaterials.2016.08.011. Epub 2016 Aug 9. Biomaterials. 2016. PMID: 27543920 Review.
Cited by
-
Metal-free atom transfer radical polymerization with ppm catalyst loading under sunlight.Nat Commun. 2021 Jan 18;12(1):429. doi: 10.1038/s41467-020-20645-8. Nat Commun. 2021. PMID: 33462235 Free PMC article.
-
New Variants of Nitroxide Mediated Polymerization.Polymers (Basel). 2020 Jul 2;12(7):1481. doi: 10.3390/polym12071481. Polymers (Basel). 2020. PMID: 32630664 Free PMC article. Review.
-
Trends in Polymers 2017/2018: Polymer Synthesis.Polymers (Basel). 2019 Dec 25;12(1):39. doi: 10.3390/polym12010039. Polymers (Basel). 2019. PMID: 31881763 Free PMC article.
-
Photoinduced Organocatalyzed Atom Transfer Radical Polymerization (O-ATRP): Precision Polymer Synthesis Using Organic Photoredox Catalysis.Chem Rev. 2022 Jan 26;122(2):1830-1874. doi: 10.1021/acs.chemrev.1c00603. Epub 2021 Nov 29. Chem Rev. 2022. PMID: 34842426 Free PMC article. Review.
-
Structural Engineering of Graphitic Carbon Nitrides for Enhanced Metal-Free PET-RAFT Polymerizations in Heterogeneous and Homogeneous Systems.ACS Omega. 2019 Sep 18;4(14):16247-16255. doi: 10.1021/acsomega.9b02597. eCollection 2019 Oct 1. ACS Omega. 2019. PMID: 31592088 Free PMC article.
References
-
- Szwarc M. “Living” Polymers. Nature. 1956;176:1168–1169. doi: 10.1038/1781168a0. - DOI
-
- Szwarc M., Levy M., Milkovich R. Polymerization Initiated by Electron Transfer Monomer. A New Method of Formation of Block Copolymers. J. Am. Chem. Soc. 1956;78:2656–2657. doi: 10.1021/ja01592a101. - DOI
-
- Pan X., Tasdelen M.A., Laun J., Junkers T., Yagci Y., Matyjaszewski K. Photomediated controlled radical polymerization. Prog. Polym. Sci. 2016;62:73–125. doi: 10.1016/j.progpolymsci.2016.06.005. - DOI
-
- Tasdelen M.A., Kahveci M.U., Yagci Y. Telechelic polymers by living and controlled/living polymerization methods. Prog. Polym. Sci. 2011;36:455–567. doi: 10.1016/j.progpolymsci.2010.10.002. - DOI
-
- Moad G., Rizzardo E. The History of Nitroxide-mediated Polymerization. In: Gigmes D., editor. Nitroxide Mediated Polymerization: From Fundamentals to Applications in Materials Science. 19th ed. Volume 19. RSC; London, UK: 2016. pp. 1–44. (RSC Polymer Series).
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

