Vascular Cell Co-Culture on Silk Fibroin Matrix
- PMID: 30966074
- PMCID: PMC6414862
- DOI: 10.3390/polym10010039
Vascular Cell Co-Culture on Silk Fibroin Matrix
Abstract
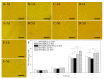
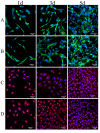
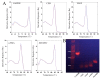
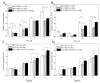
Silk fibroin (SF), a natural polymer material possessing excellent biocompatibility and biodegradability, and has been widely used in biomedical applications. In order to explore the behavior of vascular cells by co-culturing on regenerated SF matrix for use as artificial blood vessels, human aorta vascular smooth muscle cells (HAVSMCs) were co-cultured with human arterial fibroblasts (HAFs) or human umbilical vein endothelial cells (HUVECs) on SF films and SF tubular scaffolds (SFTSs). Analysis of cell morphology and deoxyribonucleic acid (DNA) content showed that HUVECs, HAVSMCs and HAFs adhered and spread well, and exhibited high proliferative activity whether cultured alone or in co-culture. Immunofluorescence and scanning electron microscopy (SEM) analysis showed that HUVECs and HAFs co-existed well with HAVSMCs on SF films or SFTSs. Cytokine expression determined by reverse transcription-polymerase chain reaction (RT-PCR) indicated that the expression levels of α-smooth muscle actin (α-SMA) and smooth muscle myosin heavy chain (SM-MHC) in HAVSMCs were inhibited on SF films or SFTSs, but expression could be obviously promoted by co-culture with HUVECs or HAFs, especially that of SM-MHC. On SF films, the expression of vascular endothelial growth factor (VEGF) and platelet endothelial cell adhesion molecule-1 (CD31) in HUVECs was promoted, and the expression levels of both increased obviously when co-cultured with HAVSMCs, with the expression levels of VEGF increasing with increasing incubation time. The expression levels of VEGF and CD31 in cells co-cultured on SFTSs improved significantly from day 3 compared with the mono-culture group. These results were beneficial to the mechanism analysis on vascular cell colonization and vascular tissue repair after in vivo transplantation of SFTSs.
Keywords: cells interaction; co-culture; proliferative activity; silk fibroin; vascular cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Steady-State Behavior and Endothelialization of a Silk-Based Small-Caliber Scaffold In Vivo Transplantation.Polymers (Basel). 2019 Aug 3;11(8):1303. doi: 10.3390/polym11081303. Polymers (Basel). 2019. PMID: 31382650 Free PMC article.
-
The effect of hirudin modification of silk fibroin on cell growth and antithrombogenicity.Mater Sci Eng C Mater Biol Appl. 2017 Jun 1;75:237-246. doi: 10.1016/j.msec.2017.02.035. Epub 2017 Feb 10. Mater Sci Eng C Mater Biol Appl. 2017. PMID: 28415459
-
Differentiation of smooth muscle progenitor cells in peripheral blood and its application in tissue engineered blood vessels.J Zhejiang Univ Sci B. 2008 Dec;9(12):923-30. doi: 10.1631/jzus.B0820257. J Zhejiang Univ Sci B. 2008. PMID: 19067459 Free PMC article.
-
Microconvex Dot-Featured Silk Fibroin Films for Promoting Human Umbilical Vein Endothelial Cell Angiogenesis via Enhancing the Expression of bFGF and VEGF.ACS Biomater Sci Eng. 2021 Jun 14;7(6):2420-2429. doi: 10.1021/acsbiomaterials.0c01647. Epub 2021 Apr 20. ACS Biomater Sci Eng. 2021. PMID: 33878261
-
Synthesis of the New-Type Vascular Endothelial Growth Factor-Silk Fibroin-Chitosan Three-Dimensional Scaffolds for Bone Tissue Engineering and In Vitro Evaluation.J Craniofac Surg. 2016 Mar;27(2):509-15. doi: 10.1097/SCS.0000000000002296. J Craniofac Surg. 2016. PMID: 26890455
Cited by
-
Developing a pro-angiogenic placenta derived amniochorionic scaffold with two exposed basement membranes as substrates for cultivating endothelial cells.Sci Rep. 2021 Nov 18;11(1):22508. doi: 10.1038/s41598-021-01922-y. Sci Rep. 2021. PMID: 34795361 Free PMC article.
-
An RGD-Containing Peptide Derived from Wild Silkworm Silk Fibroin Promotes Cell Adhesion and Spreading.Polymers (Basel). 2018 Oct 26;10(11):1193. doi: 10.3390/polym10111193. Polymers (Basel). 2018. PMID: 30961118 Free PMC article.
-
Steady-State Behavior and Endothelialization of a Silk-Based Small-Caliber Scaffold In Vivo Transplantation.Polymers (Basel). 2019 Aug 3;11(8):1303. doi: 10.3390/polym11081303. Polymers (Basel). 2019. PMID: 31382650 Free PMC article.
-
Matrix Regeneration Ability In Situ Induced by a Silk Fibroin Small-Caliber Artificial Blood Vessel In Vivo.Polymers (Basel). 2022 Sep 8;14(18):3754. doi: 10.3390/polym14183754. Polymers (Basel). 2022. PMID: 36145899 Free PMC article.
-
Enhanced Silk Fibroin/Sericin Composite Film: Preparation, Mechanical Properties and Mineralization Activity.Polymers (Basel). 2022 Jun 17;14(12):2466. doi: 10.3390/polym14122466. Polymers (Basel). 2022. PMID: 35746041 Free PMC article.
References
-
- Cummings I., George S., Kelm J., Schmidt D., Emmert M.Y., Weber B., Zünd G., Hoerstrup S.P. Tissue-engineered vascular graft remodeling in a growing lamb model: Expression of matrix metalloproteinases. Eur. J. Cardio-Thorac. Surg. 2012;41:167–172. doi: 10.1016/j.ejcts.2011.02.077. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

