Effect of Dendrigraft Generation on the Interaction between Anionic Polyelectrolytes and Dendrigraft Poly(l-Lysine)
- PMID: 30966081
- PMCID: PMC6415173
- DOI: 10.3390/polym10010045
Effect of Dendrigraft Generation on the Interaction between Anionic Polyelectrolytes and Dendrigraft Poly(l-Lysine)
Abstract
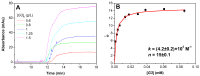
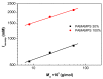
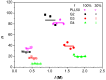
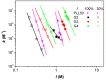
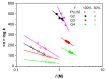
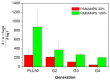
In this present work, three generations of dendrigraft poly(l-Lysine) (DGL) were studied regarding their ability to interact with linear poly (acrylamide-co-2-acrylamido-2-methyl-1-propanesulfonate) (PAMAMPS) of different chemical charge densities (30% and 100%). Frontal analysis continuous capillary electrophoresis (FACCE) was successfully applied to determine binding constants and binding stoichiometries. The effect of DGL generation on the interaction was evaluated for the first three generations (G2, G3, and G4) at different ionic strengths, and the effect of ligand topology (linear PLL vs. dendrigraft DGL) on binding parameters was evaluated. An increase of the biding site constants accompanied with a decrease of the DGL-PAMAMPS (n:1) stoichiometry was observed for increasing DGL generation. The logarithm of the global binding constants decreased linearly with the logarithm of the ionic strength. This double logarithmic representation allowed determining the extent of counter-ions released from the association of DGL molecules onto one PAMAMPS chain that was compared to the total entropic reservoir constituted by the total number of condensed counter-ions before the association.
Keywords: binding constants; counter-ion release; dendrimers; frontal analysis continuous capillary electrophoresis; ionic strength dependence; polyelectrolyte complexes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The Effect of Molar Mass and Charge Density on the Formation of Complexes between Oppositely Charged Polyelectrolytes.Polymers (Basel). 2017 Feb 4;9(2):50. doi: 10.3390/polym9020050. Polymers (Basel). 2017. PMID: 30970728 Free PMC article.
-
Modelling and predicting the interactions between oppositely and variously charged polyelectrolytes by frontal analysis continuous capillary electrophoresis.Soft Matter. 2016 Dec 6;12(48):9728-9737. doi: 10.1039/c6sm01811d. Soft Matter. 2016. PMID: 27858039
-
Effect of dendrimer generation on the interactions between human serum albumin and dendrigraft polylysines.Langmuir. 2014 Apr 22;30(15):4450-7. doi: 10.1021/la5002144. Epub 2014 Apr 7. Langmuir. 2014. PMID: 24708346
-
ε-Polylysine and next-generation dendrigraft poly-L-lysine: chemistry, activity, and applications in biopharmaceuticals.J Biomater Sci Polym Ed. 2015;26(18):1343-56. doi: 10.1080/09205063.2015.1095023. Epub 2015 Oct 27. J Biomater Sci Polym Ed. 2015. PMID: 26381379 Review.
-
[Development of a Multi-functional Nano-device for Safe and Effective Gene Delivery to Target Organs].Yakugaku Zasshi. 2016;136(11):1533-1539. doi: 10.1248/yakushi.16-00198. Yakugaku Zasshi. 2016. PMID: 27803485 Review. Japanese.
Cited by
-
Host⁻Guest Polymer Complexes.Polymers (Basel). 2018 Aug 13;10(8):911. doi: 10.3390/polym10080911. Polymers (Basel). 2018. PMID: 30960836 Free PMC article. No abstract available.
-
Molecular Dynamics of Lysine Dendrigrafts in Methanol-Water Mixtures.Int J Mol Sci. 2023 Feb 4;24(4):3063. doi: 10.3390/ijms24043063. Int J Mol Sci. 2023. PMID: 36834474 Free PMC article.
-
Study of Interactions Between Gadolinium-Based Contrast Agents and Collagen by Taylor Dispersion Analysis and Frontal Analysis Continuous Capillary Electrophoresis.Pharmaceuticals (Basel). 2024 Dec 5;17(12):1633. doi: 10.3390/ph17121633. Pharmaceuticals (Basel). 2024. PMID: 39770475 Free PMC article.
-
Understanding the Interaction of Polyelectrolyte Architectures with Proteins and Biosystems.Angew Chem Int Ed Engl. 2021 Feb 19;60(8):3882-3904. doi: 10.1002/anie.202006457. Epub 2020 Oct 27. Angew Chem Int Ed Engl. 2021. PMID: 32589355 Free PMC article. Review.
References
-
- Malkoch M., Malmström E., Nyström A.M. Dendrimers: Properties and Applications. In: Matyjaszewski K., Möller M., editors. Polymer Science: A Comprehensive Reference. Elsevier; Amsterdam, The Netherlands: 2012.
-
- Kesharwani P., Jain K., Jain N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014;39:268–307. doi: 10.1016/j.progpolymsci.2013.07.005. - DOI
LinkOut - more resources
Full Text Sources

