Formulation of Carbopol®/Poly(2-ethyl-2-oxazoline)s Mucoadhesive Tablets for Buccal Delivery of Hydrocortisone
- PMID: 30966211
- PMCID: PMC6415335
- DOI: 10.3390/polym10020175
Formulation of Carbopol®/Poly(2-ethyl-2-oxazoline)s Mucoadhesive Tablets for Buccal Delivery of Hydrocortisone
Abstract
Poly(2-ethyl-2-oxazoline) has become an excellent alternative to the use of poly(ethylene glycol) in pharmaceutical formulations due to its valuable physicochemical and biological properties. This work presents a formulation of poorly-water soluble drug, hydrocortisone, using interpolymer complexes and physical blends of poly(2-ethyl-2-oxazoline)s and two Carbopols® (Carbopol 974 and Carbopol 971) for oromucosal administration. The swelling, hydrocortisone release and mucoadhesive properties of a series of tablet formulations obtained by combination of different Carbopols with poly(2-ethyl-2-oxazoline)s of different molecular weights have been evaluated in vitro.
Keywords: Carbopol®; interpolymer complexes; mucoadhesion; poly(2-ethyl-2-oxazoline).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

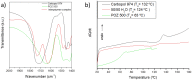
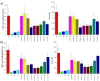



References
-
- Khadka P., Ro J., Kim H., Kim I., Kim J.T., Kim H., Cho J.M., Yun G., Lee J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014;9:304–316. doi: 10.1016/j.ajps.2014.05.005. - DOI
-
- Schulze J.D.R., Waddington W.A., Ell P.J., Parsons G.E., Coffin M.D., Basit A.W. Concentration-Dependent Effects of Polyethylene Glycol 400 on Gastrointestinal Transit and Drug Absorption. Pharm. Res. 2003;20:1984–1988. doi: 10.1023/B:PHAM.0000008046.64409.bd. - DOI - PubMed
-
- Harris J.M., Dust J.M., McGill R.A., Harris P.A., Edgell M.J., Sedaghat-Herati R.M., Karr L.J., Donnelly D.L. Water-Soluble Polymers. Volume 467. American Chemical Society; Washington, DC, USA: 1991. New Polyethylene Glycols for Biomedical Applications; pp. 27–418. (ACS Symposium Series).
LinkOut - more resources
Full Text Sources

