3D Printing and Electrospinning of Composite Hydrogels for Cartilage and Bone Tissue Engineering
- PMID: 30966320
- PMCID: PMC6414880
- DOI: 10.3390/polym10030285
3D Printing and Electrospinning of Composite Hydrogels for Cartilage and Bone Tissue Engineering
Abstract
Injuries of bone and cartilage constitute important health issues costing the National Health Service billions of pounds annually, in the UK only. Moreover, these damages can become cause of disability and loss of function for the patients with associated social costs and diminished quality of life. The biomechanical properties of these two tissues are massively different from each other and they are not uniform within the same tissue due to the specific anatomic location and function. In this perspective, tissue engineering (TE) has emerged as a promising approach to address the complexities associated with bone and cartilage regeneration. Tissue engineering aims at developing temporary three-dimensional multicomponent constructs to promote the natural healing process. Biomaterials, such as hydrogels, are currently extensively studied for their ability to reproduce both the ideal 3D extracellular environment for tissue growth and to have adequate mechanical properties for load bearing. This review will focus on the use of two manufacturing techniques, namely electrospinning and 3D printing, that present promise in the fabrication of complex composite gels for cartilage and bone tissue engineering applications.
Keywords: 3D printing; bone; cartilage; composite hydrogels; electrospinning.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures




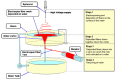


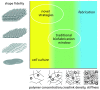
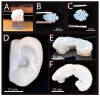

References
-
- Kim T.G., Shin H., Lim D.W. Biomimetic Scaffolds for Tissue Engineering. Adv. Funct. Mater. 2012;22:2446–2468. doi: 10.1002/adfm.201103083. - DOI
Publication types
LinkOut - more resources
Full Text Sources

