Ultrasound- and Thermo-Responsive Ionic Liquid Polymers
- PMID: 30966336
- PMCID: PMC6415017
- DOI: 10.3390/polym10030301
Ultrasound- and Thermo-Responsive Ionic Liquid Polymers
Abstract
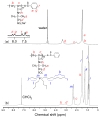
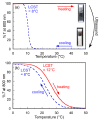
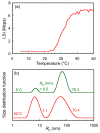
Poly(sodium 2-acrylamido-2-methylpropanesulfonate) (PAMPSNa) was prepared via reversible addition-fragmentation chain transfer (RAFT) radical polymerization. An ionic liquid polymer (PAMPSP4448) was then prepared by exchanging the pendant counter cation from sodium (Na⁺) to tributyl-n-octylphosphonium (P4448⁺). We studied the ultrasound- and thermo-responsive behaviors of PAMPSP4448 in water. When the aqueous PAMPSP4448 solution was heated from 5 to 50 °C, the solution was always transparent with 100% transmittance. Unimers and interpolymer aggregates coexisted in water in the temperature range 5⁻50 °C. Generally, hydrogen bonding interactions are broken as the temperature increases due to increased molecular motion. Above 25 °C, the size of the interpolymer aggregates decreased, because hydrophobic interactions inside them were strengthened by dehydration accompanying cleavage of hydrogen bonds between water molecules and the pendant amide or sulfonate groups in PAMPSP4448. Above 25 °C, sonication of the aqueous solution induced an increase in the collision frequency of the aggregates. This promoted hydrophobic interactions between the aggregates to form larger aggregates, and the aqueous solution became turbid. When the temperature was decreased below 8 °C, hydrogen bonds reformed between water molecules and the pendant amide or sulfonate groups, allowing PAMPSP4448 to redissolve in water to form a transparent solution. The solution could be repeatedly controlled between turbidity and transparency by sonication and cooling, respectively.
Keywords: electrostatic repulsion; hydrophobic interaction; ionic liquid polymer; lower critical solution temperature (LCST); reversible addition-fragmentation chain transfer (RAFT) polymerization; thermo-responsive; ultrasound.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources

