Reusable Xerogel Containing Quantum Dots with High Fluorescence Retention
- PMID: 30966345
- PMCID: PMC6414965
- DOI: 10.3390/polym10030310
Reusable Xerogel Containing Quantum Dots with High Fluorescence Retention
Abstract
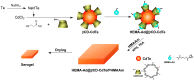
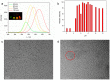
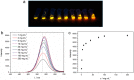
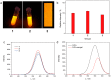
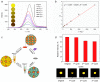
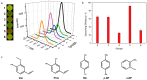
Although various analytical methods have been established based on quantum dots (QDs), most were conducted in solution, which is inadequate for storage/transportation and rapid analysis. Moreover, the potential environmental problems caused by abandoned QDs cannot be ignored. In this paper, a reusable xerogel containing CdTe with strong emission is established by introducing host⁻guest interactions between QDs and polymer matrix. This xerogel shows high QDs loading capacity without decrease or redshift in fluorescence (the maximum of loading is 50 wt % of the final xerogel), which benefits from the steric hindrance of β-cyclodextrin (βCD) molecules. Host⁻guest interactions immobilize QDs firmly, resulting in the excellent fluorescence retention of the xerogel. The good detecting performance and reusability mean this xerogel could be employed as a versatile analysis platform (for quantitative and qualitative analyses). In addition, the xerogel can be self-healed by the aid of water.
Keywords: host–guest interactions; quantum dots; xerogel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Hildebrandt N., Spillmann C.M., Algar W.R., Pons T., Stewart M.H., Oh E., Susumu K., Díaz S.A., Delehanty J.B., Medintz I.L. Energy Transfer with Semiconductor Quantum Dot Bioconjugates: A Versatile Platform for Biosensing, Energy Harvesting, and Other Developing Applications. Chem. Rev. 2017;117:536–711. doi: 10.1021/acs.chemrev.6b00030. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

