Aliphatic Polyester Nanofibers Functionalized with Cyclodextrins and Cyclodextrin-Guest Inclusion Complexes
- PMID: 30966463
- PMCID: PMC6415270
- DOI: 10.3390/polym10040428
Aliphatic Polyester Nanofibers Functionalized with Cyclodextrins and Cyclodextrin-Guest Inclusion Complexes
Abstract
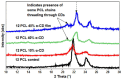
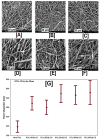
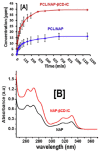
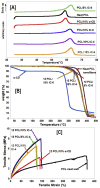
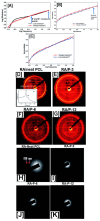
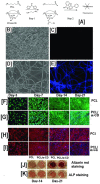
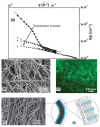
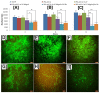
The fabrication of nanofibers by electrospinning has gained popularity in the past two decades; however, only in this decade, have polymeric nanofibers been functionalized using cyclodextrins (CDs) or their inclusion complexes (ICs). By combining electrospinning of polymers with free CDs, nanofibers can be fabricated that are capable of capturing small molecules, such as wound odors or environmental toxins in water and air. Likewise, combining polymers with cyclodextrin-inclusion complexes (CD-ICs), has shown promise in enhancing or controlling the delivery of small molecule guests, by minor tweaking in the technique utilized in fabricating these nanofibers, for example, by forming core⁻shell or multilayered structures and conventional electrospinning, for controlled and rapid delivery, respectively. In addition to small molecule delivery, the thermomechanical properties of the polymers can be significantly improved, as our group has shown recently, by adding non-stoichiometric inclusion complexes to the polymeric nanofibers. We recently reported and thoroughly characterized the fabrication of polypseudorotaxane (PpR) nanofibers without a polymeric carrier. These PpR nanofibers show unusual rheological and thermomechanical properties, even when the coverage of those polymer chains is relatively sparse (~3%). A key advantage of these PpR nanofibers is the presence of relatively stable hydroxyl groups on the outer surface of the nanofibers, which can subsequently be taken advantage of for bioconjugation, making them suitable for biomedical applications. Although the number of studies in this area is limited, initial results suggest significant potential for bone tissue engineering, and with additional bioconjugation in other areas of tissue engineering. In addition, the behaviors and uses of aliphatic polyester nanofibers functionalized with CDs and CD-ICs are briefly described and summarized. Based on these observations, we attempt to draw conclusions for each of these combinations, and the relationships that exist between their presence and the functional behaviors of their nanofibers.
Keywords: controlled drug delivery; cyclodextrin-inclusion complexes; cyclodextrins; mechanical properties; poly(lactic acid); poly(ε-caprolactone); pseudorotaxanes; rapid dissolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Kny E., Uyar T., Kny E. In: Electrospun Materials for Tissue Engineering and Biomedical Applications. Kny E., Uyar T., editors. Woodhead Publishing; Sawston, UK: 2017. pp. i–iii.
-
- Anu Bhushani J., Anandharamakrishnan C. Electrospinning and electrospraying techniques: Potential food based applications. Trends Food Sci. Technol. 2014;38:21–33. doi: 10.1016/j.tifs.2014.03.004. - DOI
-
- Wang W., Huang H., Li Z., Zhang H., Wang Y., Zheng W., Wang C. Zinc Oxide Nanofiber Gas Sensors via Electrospinning. J. Am. Ceram. Soc. 2008;91:3817–3819. doi: 10.1111/j.1551-2916.2008.02765.x. - DOI
-
- Ramakrishna S., Fujihara K., Teo W.-E., Yong T., Ma Z., Ramaseshan R. Electrospun nanofibers: Solving global issues. Mater. Today. 2006;9:40–50. doi: 10.1016/S1369-7021(06)71389-X. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

