Chitosan in Non-Viral Gene Delivery: Role of Structure, Characterization Methods, and Insights in Cancer and Rare Diseases Therapies
- PMID: 30966479
- PMCID: PMC6415274
- DOI: 10.3390/polym10040444
Chitosan in Non-Viral Gene Delivery: Role of Structure, Characterization Methods, and Insights in Cancer and Rare Diseases Therapies
Abstract
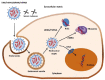
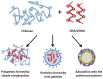
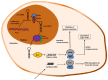
Non-viral gene delivery vectors have lagged far behind viral ones in the current pipeline of clinical trials of gene therapy nanomedicines. Even when non-viral nanovectors pose less safety risks than do viruses, their efficacy is much lower. Since the early studies to deliver pDNA, chitosan has been regarded as a highly attractive biopolymer to deliver nucleic acids intracellularly and induce a transgenic response resulting in either upregulation of protein expression (for pDNA, mRNA) or its downregulation (for siRNA or microRNA). This is explained as the consequence of a multi-step process involving condensation of nucleic acids, protection against degradation, stabilization in physiological conditions, cellular internalization, release from the endolysosome ("proton sponge" effect), unpacking and enabling the trafficking of pDNA to the nucleus or the siRNA to the RNA interference silencing complex (RISC). Given the multiple steps and complexity involved in the gene transfection process, there is a dearth of understanding of the role of chitosan's structural features (Mw and degree of acetylation, DA%) on each step that dictates the net transfection efficiency and its kinetics. The use of fully characterized chitosan samples along with the utilization of complementary biophysical and biological techniques is key to bridging this gap of knowledge and identifying the optimal chitosans for delivering a specific gene. Other aspects such as cell type and administration route are also at play. At the same time, the role of chitosan structural features on the morphology, size and surface composition of synthetic virus-like particles has barely been addressed. The ongoing revolution brought about by the recent discovery of CRISPR-Cas9 technology will undoubtedly be a game changer in this field in the short term. In the field of rare diseases, gene therapy is perhaps where the greatest potential lies and we anticipate that chitosans will be key players in the translation of research to the clinic.
Keywords: chitosan structure; gene delivery; non-viral vectors; pDNA; siRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Phosphorylatable short peptide conjugated low molecular weight chitosan for efficient siRNA delivery and target gene silencing.Int J Pharm. 2012 Jan 17;422(1-2):445-53. doi: 10.1016/j.ijpharm.2011.10.041. Epub 2011 Oct 28. Int J Pharm. 2012. PMID: 22067703
-
Chitosans for delivery of nucleic acids.Adv Drug Deliv Rev. 2013 Aug;65(9):1234-70. doi: 10.1016/j.addr.2013.07.005. Epub 2013 Jul 18. Adv Drug Deliv Rev. 2013. PMID: 23872012 Free PMC article. Review.
-
Chitosan nanoparticles as non-viral gene delivery vehicles based on atomic force microscopy study.Acta Biochim Biophys Sin (Shanghai). 2009 Jun;41(6):515-26. doi: 10.1093/abbs/gmp038. Acta Biochim Biophys Sin (Shanghai). 2009. PMID: 19499155
-
The mechanism of naked DNA uptake and expression.Adv Genet. 2005;54:3-20. doi: 10.1016/S0065-2660(05)54001-X. Adv Genet. 2005. PMID: 16096005 Review.
-
Biophysical properties of chitosan/siRNA polyplexes: profiling the polymer/siRNA interactions and bioactivity.J Control Release. 2012 Jan 30;157(2):297-304. doi: 10.1016/j.jconrel.2011.08.023. Epub 2011 Aug 22. J Control Release. 2012. PMID: 21884740
Cited by
-
Gene Therapy in Rare Respiratory Diseases: What Have We Learned So Far?J Clin Med. 2020 Aug 8;9(8):2577. doi: 10.3390/jcm9082577. J Clin Med. 2020. PMID: 32784514 Free PMC article. Review.
-
Broadening the Horizons of RNA Delivery Strategies in Cancer Therapy.Bioengineering (Basel). 2022 Oct 19;9(10):576. doi: 10.3390/bioengineering9100576. Bioengineering (Basel). 2022. PMID: 36290544 Free PMC article. Review.
-
Genetically Engineered-MSC Therapies for Non-unions, Delayed Unions and Critical-size Bone Defects.Int J Mol Sci. 2019 Jul 12;20(14):3430. doi: 10.3390/ijms20143430. Int J Mol Sci. 2019. PMID: 31336890 Free PMC article. Review.
-
Research Status and Prospect of Non-Viral Vectors Based on siRNA: A Review.Int J Mol Sci. 2023 Feb 8;24(4):3375. doi: 10.3390/ijms24043375. Int J Mol Sci. 2023. PMID: 36834783 Free PMC article. Review.
-
siRNA delivery using intelligent chitosan-capped mesoporous silica nanoparticles for overcoming multidrug resistance in malignant carcinoma cells.Sci Rep. 2021 Oct 15;11(1):20531. doi: 10.1038/s41598-021-00085-0. Sci Rep. 2021. PMID: 34654836 Free PMC article.
References
-
- Tibbals H.F. Medical Nanotechnology and Nanomedicine. CRC Press; Boca Raton, FL, USA: 2010.
-
- Azzouz M., Martin-Rendon E., Barber R.D., Mitrophanous K.A., Carter E.E., Rohll J.B., Kingsman S.M., Kingsman A.J., Mazarakis N.D. Multicistronic Lentiviral Vector-Mediated Striatal Gene Transfer of Aromatic l-Amino Acid Decarboxylase, Tyrosine Hydroxylase, and GTP Cyclohydrolase I Induces Sustained Transgene Expression, Dopamine Production, and Functional Improvement in a Rat Model. J. Neurosci. 2002;22:10302–10312. doi: 10.1523/JNEUROSCI.22-23-10302.2002. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

