New Three-Dimensional Poly(decanediol-co-tricarballylate) Elastomeric Fibrous Mesh Fabricated by Photoreactive Electrospinning for Cardiac Tissue Engineering Applications
- PMID: 30966490
- PMCID: PMC6415264
- DOI: 10.3390/polym10040455
New Three-Dimensional Poly(decanediol-co-tricarballylate) Elastomeric Fibrous Mesh Fabricated by Photoreactive Electrospinning for Cardiac Tissue Engineering Applications
Abstract
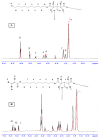
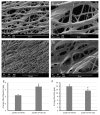
Reactive electrospinning is capable of efficiently producing in situ crosslinked scaffolds resembling the natural extracellular matrix with tunable characteristics. In this study, we aimed to synthesize, characterize, and investigate the in vitro cytocompatibility of electrospun fibers of acrylated poly(1,10-decanediol-co-tricarballylate) copolymer prepared utilizing the photoreactive electrospinning process with ultraviolet radiation for crosslinking, to be used for cardiac tissue engineering applications. Chemical, thermal, and morphological characterization confirmed the successful synthesis of the polymer used for production of the electrospun fibrous scaffolds with more than 70% porosity. Mechanical testing confirmed the elastomeric nature of the fibers required to withstand cardiac contraction and relaxation. The cell viability assay showed no significant cytotoxicity of the fibers on cultured cardiomyoblasts and the cell-scaffolds interaction study showed a significant increase in cell attachment and growth on the electrospun fibers compared to the reference. This data suggests that the newly synthesized fibrous scaffold constitutes a promising candidate for cardiac tissue engineering applications.
Keywords: biocompatibility; cardiac tissue engineering; particulate leaching; photo-crosslinking; poly(diol-tricarballylate); reactive electrospinning.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- GUSTO Angiographic Investigators The effects of tissue plasminogen activator, streptokinase, or both on coronary-artery patency, ventricular function, and survival after acute myocardial infarction. N. Engl. J. Med. 1993;329:1615–1622. - PubMed
-
- Cheng Y., Yi G., Conditt G.B., Sheehy A., Kolodgie F.D., Tellez A., Polyakov I., Gu A., Aboodi M.S., Wallace-Bradley D., et al. Catheter-based endomyocardial delivery of mesenchymal precursor cells using 3d echo guidance improves cardiac function in a chronic myocardial injury ovine model. Cell Trans. 2013;22:2299–2309. doi: 10.3727/096368912X658016. - DOI - PubMed
-
- Ripa R.S., Jorgensen E., Wang Y., Thune J.J., Nilsson J.C., Sondergaard L., Johnsen H.E., Kober L., Grande P., Kastrup J. Stem cell mobilization induced by subcutaneous granulocyte-colony stimulating factor to improve cardiac regeneration after acute st-elevation myocardial infarction: Result of the double-blind, randomized, placebo-controlled stem cells in myocardial infarction (stemmi) trial. Circulation. 2006;113:1983–1992. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

