Mechanisms, Detection, and Relevance of Protein Acetylation in Prokaryotes
- PMID: 30967470
- PMCID: PMC6456759
- DOI: 10.1128/mBio.02708-18
Mechanisms, Detection, and Relevance of Protein Acetylation in Prokaryotes
Abstract
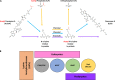
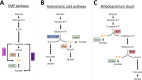
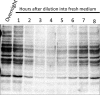
Posttranslational modification of a protein, either alone or in combination with other modifications, can control properties of that protein, such as enzymatic activity, localization, stability, or interactions with other molecules. N-ε-Lysine acetylation is one such modification that has gained attention in recent years, with a prevalence and significance that rival those of phosphorylation. This review will discuss the current state of the field in bacteria and some of the work in archaea, focusing on both mechanisms of N-ε-lysine acetylation and methods to identify, quantify, and characterize specific acetyllysines. Bacterial N-ε-lysine acetylation depends on both enzymatic and nonenzymatic mechanisms of acetylation, and recent work has shed light into the regulation of both mechanisms. Technological advances in mass spectrometry have allowed researchers to gain insight with greater biological context by both (i) analyzing samples either with stable isotope labeling workflows or using label-free protocols and (ii) determining the true extent of acetylation on a protein population through stoichiometry measurements. Identification of acetylated lysines through these methods has led to studies that probe the biological significance of acetylation. General and diverse approaches used to determine the effect of acetylation on a specific lysine will be covered.
Keywords: acetylation; acetylome; bacteria; lysine acetyltransferase; mass spectrometry; proteomics.
Copyright © 2019 Christensen et al.
Figures

References
-
- Nakayasu ES, Burnet MC, Walukiewicz HE, Wilkins CS, Shukla AK, Brooks S, Plutz MJ, Lee BD, Schilling B, Wolfe AJ, Müller S, Kirby JR, Rao CV, Cort JR, Payne SH. 2017. Ancient regulatory role of lysine acetylation in central metabolism. mBio 8:e01894-17. doi: 10.1128/mBio.01894-17. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
