Accuracy of Patient Health Questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis
- PMID: 30967483
- PMCID: PMC6454318
- DOI: 10.1136/bmj.l1476
Accuracy of Patient Health Questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis
Erratum in
-
Accuracy of Patient Health Questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis.BMJ. 2019 Apr 12;365:l1781. doi: 10.1136/bmj.l1781. BMJ. 2019. PMID: 30979729 Free PMC article. No abstract available.
Abstract
Objective: To determine the accuracy of the Patient Health Questionnaire-9 (PHQ-9) for screening to detect major depression.
Design: Individual participant data meta-analysis.
Data sources: Medline, Medline In-Process and Other Non-Indexed Citations, PsycINFO, and Web of Science (January 2000-February 2015).
Inclusion criteria: Eligible studies compared PHQ-9 scores with major depression diagnoses from validated diagnostic interviews. Primary study data and study level data extracted from primary reports were synthesized. For PHQ-9 cut-off scores 5-15, bivariate random effects meta-analysis was used to estimate pooled sensitivity and specificity, separately, among studies that used semistructured diagnostic interviews, which are designed for administration by clinicians; fully structured interviews, which are designed for lay administration; and the Mini International Neuropsychiatric (MINI) diagnostic interviews, a brief fully structured interview. Sensitivity and specificity were examined among participant subgroups and, separately, using meta-regression, considering all subgroup variables in a single model.
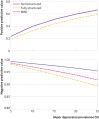
Results: Data were obtained for 58 of 72 eligible studies (total n=17 357; major depression cases n=2312). Combined sensitivity and specificity was maximized at a cut-off score of 10 or above among studies using a semistructured interview (29 studies, 6725 participants; sensitivity 0.88, 95% confidence interval 0.83 to 0.92; specificity 0.85, 0.82 to 0.88). Across cut-off scores 5-15, sensitivity with semistructured interviews was 5-22% higher than for fully structured interviews (MINI excluded; 14 studies, 7680 participants) and 2-15% higher than for the MINI (15 studies, 2952 participants). Specificity was similar across diagnostic interviews. The PHQ-9 seems to be similarly sensitive but may be less specific for younger patients than for older patients; a cut-off score of 10 or above can be used regardless of age..
Conclusions: PHQ-9 sensitivity compared with semistructured diagnostic interviews was greater than in previous conventional meta-analyses that combined reference standards. A cut-off score of 10 or above maximized combined sensitivity and specificity overall and for subgroups.
Registration: PROSPERO CRD42014010673.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICJME uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work other than that described above; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years with the following exceptions: NJ and SP received a grant, outside the submitted work, from the University of Calgary Hotchkiss Brain Institute, which was jointly funded by the Institute and Pfizer; Pfizer was the original sponsor of the development of the PHQ-9, which is now in the public domain; JCNC is a steering committee member or consultant of Astra Zeneca, Bayer, Lilly, MSD, and Pfizer and has received sponsorships and honorariums for giving lectures and providing consultancy, and her affiliated institution has received research grants from these companies; UH was an advisory board member for Lundbeck and Servier, a consultant for Bayer Pharma, and a speaker for Roche Pharma and Servier and has received personal fees from Janssen, all outside the submitted work; MI has received a grant from Novartis Pharma and personal fees from Meiji, Mochida, Takeda, Novartis, Yoshitomi, Pfizer, Eisai, Otsuka, MSD, Technomics, and Sumitomo Dainippon, all outside of the submitted work; no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann 2002;32:1-7 10.3928/0048-5713-20020901-06. - DOI
Publication types
MeSH terms
Grants and funding
- R01 HD039415/HD/NICHD NIH HHS/United States
- T37 MD001449/MD/NIMHD NIH HHS/United States
- R34 MH072925/MH/NIMH NIH HHS/United States
- K02 MH065919/MH/NIMH NIH HHS/United States
- P30 DK050456/DK/NIDDK NIH HHS/United States
- R24 MH056587/MH/NIMH NIH HHS/United States
- R01 MH073687/MH/NIMH NIH HHS/United States
- R34 MH084673/MH/NIMH NIH HHS/United States
- T32 GM007356/GM/NIGMS NIH HHS/United States
- R36 HS018246/HS/AHRQ HHS/United States
- R24 MH071604/MH/NIMH NIH HHS/United States
- TL1 RR024135/RR/NCRR NIH HHS/United States
- R01 MH069666/MH/NIMH NIH HHS/United States
- R01 HL079235/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
