Antidepressants affect gut microbiota and Ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior
- PMID: 30967529
- PMCID: PMC6456569
- DOI: 10.1038/s41398-019-0466-x
Antidepressants affect gut microbiota and Ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior
Abstract
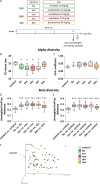
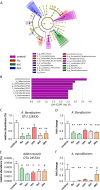
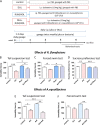
Accumulating evidence demonstrates that the gut microbiota affects brain function and behavior, including depressive behavior. Antidepressants are the main drugs used for treatment of depression. We hypothesized that antidepressant treatment could modify gut microbiota which can partially mediate their antidepressant effects. Mice were chronically treated with one of five antidepressants (fluoxetine, escitalopram, venlafaxine, duloxetine or desipramine), and gut microbiota was analyzed, using 16s rRNA gene sequencing. After characterization of differences in the microbiota, chosen bacterial species were supplemented to vehicle and antidepressant-treated mice, and depressive-like behavior was assessed to determine bacterial effects. RNA-seq analysis was performed to determine effects of bacterial treatment in the brain. Antidepressants reduced richness and increased beta diversity of gut bacteria, compared to controls. At the genus level, antidepressants reduced abundances of Ruminococcus, Adlercreutzia, and an unclassified Alphaproteobacteria. To examine implications of the dysregulated bacteria, we chose one of antidepressants (duloxetine) and investigated if its antidepressive effects can be attenuated by simultaneous treatment with Ruminococcus flavefaciens or Adlercreutzia equolifaciens. Supplementation with R. flavefaciens diminished duloxetine-induced decrease in depressive-like behavior, while A. equolifaciens had no such effect. R. flavefaciens treatment induced changes in cortical gene expression, up-regulating genes involved in mitochondrial oxidative phosphorylation, while down-regulating genes involved in neuronal plasticity. Our results demonstrate that various types of antidepressants alter gut microbiota composition, and further implicate a role for R. flavefaciens in alleviating depressive-like behavior. Moreover, R. flavefaciens affects gene networks in the brain, suggesting a mechanism for microbial regulation of antidepressant treatment efficiency.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Chinese medicine formula Kai-Xin-San ameliorates depression-like behaviours in chronic unpredictable mild stressed mice by regulating gut microbiota-inflammation-stress system.J Ethnopharmacol. 2020 Oct 28;261:113055. doi: 10.1016/j.jep.2020.113055. Epub 2020 Jun 24. J Ethnopharmacol. 2020. PMID: 32592887
-
Abnormal gut microbiota composition is associated with experimental autoimmune prostatitis-induced depressive-like behaviors in mice.Prostate. 2020 Jun;80(9):663-673. doi: 10.1002/pros.23978. Epub 2020 Apr 7. Prostate. 2020. PMID: 32255522
-
Antidepressant drugs promote the spread of broad-host-range plasmid in mouse and human gut microbiota.Gut Microbes. 2025 Dec;17(1):2514138. doi: 10.1080/19490976.2025.2514138. Epub 2025 Jun 3. Gut Microbes. 2025. PMID: 40462285 Free PMC article.
-
Antidepressants, antimicrobials or both? Gut microbiota dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness.J Affect Disord. 2017 Jan 15;208:22-32. doi: 10.1016/j.jad.2016.09.012. Epub 2016 Sep 28. J Affect Disord. 2017. PMID: 27744123 Review.
-
The bidirectional interaction between antidepressants and the gut microbiota: are there implications for treatment response?Int Clin Psychopharmacol. 2025 Jan 1;40(1):3-26. doi: 10.1097/YIC.0000000000000533. Epub 2024 Feb 6. Int Clin Psychopharmacol. 2025. PMID: 39621492 Free PMC article. Review.
Cited by
-
Beneficial Effect of Alkaloids From Sophora alopecuroides L. on CUMS-Induced Depression Model Mice via Modulating Gut Microbiota.Front Cell Infect Microbiol. 2021 Apr 19;11:665159. doi: 10.3389/fcimb.2021.665159. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33954123 Free PMC article.
-
Interactions between maternal fluoxetine exposure, the maternal gut microbiome and fetal neurodevelopment in mice.Behav Brain Res. 2021 Jul 23;410:113353. doi: 10.1016/j.bbr.2021.113353. Epub 2021 May 9. Behav Brain Res. 2021. PMID: 33979656 Free PMC article.
-
Clock gene Per3 deficiency disrupts circadian alterations of gut microbiota in mice.Acta Biochim Biophys Sin (Shanghai). 2023 Dec 25;55(12):2004-2007. doi: 10.3724/abbs.2023257. Acta Biochim Biophys Sin (Shanghai). 2023. PMID: 37964605 Free PMC article. No abstract available.
-
Depression and the Aberrant Intestinal Microbiome.Gastroenterol Hepatol (N Y). 2024 Jan;20(1):30-40. Gastroenterol Hepatol (N Y). 2024. PMID: 38405047 Free PMC article.
-
Characterization of gut microbiome in mice model of depression with divergent response to escitalopram treatment.Transl Psychiatry. 2021 May 20;11(1):303. doi: 10.1038/s41398-021-01428-1. Transl Psychiatry. 2021. PMID: 34016954 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

