Deletion of Dtnbp1 in mice impairs threat memory consolidation and is associated with enhanced inhibitory drive in the amygdala
- PMID: 30967545
- PMCID: PMC6456574
- DOI: 10.1038/s41398-019-0465-y
Deletion of Dtnbp1 in mice impairs threat memory consolidation and is associated with enhanced inhibitory drive in the amygdala
Abstract
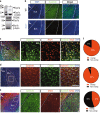
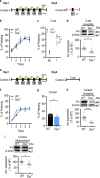
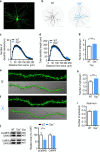
Schizophrenia is a severe and highly heritable disorder. Dystrobrevin-binding protein 1 (DTNBP1), also known as dysbindin-1, has been implicated in the pathophysiology of schizophrenia. Specifically, dysbindin-1 mRNA and protein expression are decreased in the brains of subjects with this disorder. Mice lacking dysbinidn-1 also display behavioral phenotypes similar to those observed in schizophrenic patients. However, it remains unknown whether deletion of dysbindin-1 impacts functions of the amygdala, a brain region that is critical for emotional processing, which is disrupted in patients with schizophrenia. Here, we show that dysbindin-1 is expressed in both excitatory and inhibitory neurons of the basolateral amygdala (BLA). Deletion of dysbindin-1 in male mice (Dys-/-) impaired cued and context-dependent threat memory, without changes in measures of anxiety. The behavioral deficits observed in Dys-/- mice were associated with perturbations in the BLA, including the enhancement of GABAergic inhibition of pyramidal neurons, increased numbers of parvalbumin interneurons, and morphological abnormalities of dendritic spines on pyramidal neurons. Our findings highlight an important role for dysbindin-1 in the regulation of amygdalar function and indicate that enhanced inhibition of BLA pyramidal neuron activity may contribute to the weakened threat memory expression observed in Dys-/- mice.
Conflict of interest statement
D.T.B. served as a consultant for LifeSci Capital. The remaining authors declare that they have no conflict of interest.
Figures


Similar articles
-
BDNF rescues prefrontal dysfunction elicited by pyramidal neuron-specific DTNBP1 deletion in vivo.J Mol Cell Biol. 2017 Apr 1;9(2):117-131. doi: 10.1093/jmcb/mjw029. J Mol Cell Biol. 2017. PMID: 27330059 Free PMC article.
-
Acute stress in adolescence vs early adulthood following selective deletion of dysbindin-1A: Effects on anxiety, cognition and other schizophrenia-related phenotypes.J Psychopharmacol. 2019 Dec;33(12):1610-1619. doi: 10.1177/0269881119875465. Epub 2019 Sep 26. J Psychopharmacol. 2019. PMID: 31556815
-
Single point mutation on the gene encoding dysbindin results in recognition deficits.Genes Brain Behav. 2018 Jun;17(5):e12449. doi: 10.1111/gbb.12449. Epub 2018 Jan 11. Genes Brain Behav. 2018. PMID: 29227583
-
[Risk genes for schizophrenia and neuronal plasticity: molecular target for antipsychotic discovery].Nihon Shinkei Seishin Yakurigaku Zasshi. 2010 Jun;30(3):103-7. Nihon Shinkei Seishin Yakurigaku Zasshi. 2010. PMID: 20666140 Review. Japanese.
-
The sandy (sdy) mouse: a dysbindin-1 mutant relevant to schizophrenia research.Prog Brain Res. 2009;179:87-94. doi: 10.1016/S0079-6123(09)17910-4. Epub 2009 Nov 20. Prog Brain Res. 2009. PMID: 20302821 Review.
Cited by
-
Transcriptomics analysis reveals potential regulatory role of nSMase2 (Smpd3) in nervous system development and function of middle-aged mouse brains.Genes Brain Behav. 2024 Aug;23(4):e12911. doi: 10.1111/gbb.12911. Genes Brain Behav. 2024. PMID: 39171374 Free PMC article.
-
Loss of dysbindin-1 affects GABAergic transmission in the PFC.Psychopharmacology (Berl). 2019 Nov;236(11):3291-3300. doi: 10.1007/s00213-019-05285-1. Epub 2019 Jun 14. Psychopharmacology (Berl). 2019. PMID: 31201475 Free PMC article.
-
Sex dimorphism controls dysbindin-related cognitive dysfunctions in mice and humans with the contribution of COMT.Mol Psychiatry. 2024 Sep;29(9):2666-2677. doi: 10.1038/s41380-024-02527-3. Epub 2024 Mar 26. Mol Psychiatry. 2024. PMID: 38532008 Free PMC article.
-
Psychosocial stress and cannabinoid drugs affect acetylation of α-tubulin (K40) and gene expression in the prefrontal cortex of adult mice.PLoS One. 2022 Sep 21;17(9):e0274352. doi: 10.1371/journal.pone.0274352. eCollection 2022. PLoS One. 2022. PMID: 36129937 Free PMC article.
-
Synapse-specific roles for microglia in development: New horizons in the prefrontal cortex.Front Mol Neurosci. 2022 Aug 8;15:965756. doi: 10.3389/fnmol.2022.965756. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36003220 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

