Therapeutic targeting of trained immunity
- PMID: 30967658
- PMCID: PMC7069501
- DOI: 10.1038/s41573-019-0025-4
Therapeutic targeting of trained immunity
Abstract
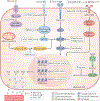
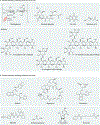
Immunotherapy is revolutionizing the treatment of diseases in which dysregulated immune responses have an important role. However, most of the immunotherapy strategies currently being developed engage the adaptive immune system. In the past decade, both myeloid (monocytes, macrophages and dendritic cells) and lymphoid (natural killer cells and innate lymphoid cells) cell populations of the innate immune system have been shown to display long-term changes in their functional programme through metabolic and epigenetic programming. Such reprogramming causes these cells to be either hyperresponsive or hyporesponsive, resulting in a changed immune response to secondary stimuli. This de facto innate immune memory, which has been termed 'trained immunity', provides a powerful 'targeting framework' to regulate the delicate balance of immune homeostasis, priming, training and tolerance. In this Opinion article, we set out our vision of how to target innate immune cells and regulate trained immunity to achieve long-term therapeutic benefits in a range of immune-related diseases. These include conditions characterized by excessive trained immunity, such as inflammatory and autoimmune disorders, allergies and cardiovascular disease and conditions driven by defective trained immunity, such as cancer and certain infections.
Conflict of interest statement
Competing interests
The authors declare that they are scientific founders of Trained Therapeutics Discovery.
Figures
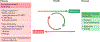
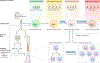

References
-
- Lesterhuis WJ, Haanen JBAG & Punt CJA Cancer immunotherapy - revisited. Nat. Rev Drug Discov 10, 591–600 (2011). - PubMed
-
- Tontonoz M Immunotherapy: revolutionizing cancer treatment since 1891. MSKCC.org. https://www.mskcc.org/blog/immunotherapy-revolutionizing-cancer-treatmen... (2015).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

