Resistance Toward Chlorhexidine in Oral Bacteria - Is There Cause for Concern?
- PMID: 30967854
- PMCID: PMC6439480
- DOI: 10.3389/fmicb.2019.00587
Resistance Toward Chlorhexidine in Oral Bacteria - Is There Cause for Concern?
Abstract
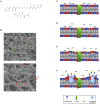
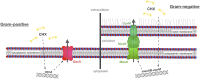
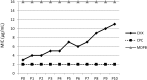
The threat of antibiotic resistance has attracted strong interest during the last two decades, thus stimulating stewardship programs and research on alternative antimicrobial therapies. Conversely, much less attention has been given to the directly related problem of resistance toward antiseptics and biocides. While bacterial resistances toward triclosan or quaternary ammonium compounds have been considered in this context, the bis-biguanide chlorhexidine (CHX) has been put into focus only very recently when its use was associated with emergence of stable resistance to the last-resort antibiotic colistin. The antimicrobial effect of CHX is based on damaging the bacterial cytoplasmic membrane and subsequent leakage of cytoplasmic material. Consequently, mechanisms conferring resistance toward CHX include multidrug efflux pumps and cell membrane changes. For instance, in staphylococci it has been shown that plasmid-borne qac ("quaternary ammonium compound") genes encode Qac efflux proteins that recognize cationic antiseptics as substrates. In Pseudomonas stutzeri, changes in the outer membrane protein and lipopolysaccharide profiles have been implicated in CHX resistance. However, little is known about the risk of resistance toward CHX in oral bacteria and potential mechanisms conferring this resistance or even cross-resistances toward antibiotics. Interestingly, there is also little awareness about the risk of CHX resistance in the dental community even though CHX has been widely used in dental practice as the gold-standard antiseptic for more than 40 years and is also included in a wide range of oral care consumer products. This review provides an overview of general resistance mechanisms toward CHX and the evidence for CHX resistance in oral bacteria. Furthermore, this work aims to raise awareness among the dental community about the risk of resistance toward CHX and accompanying cross-resistance to antibiotics. We propose new research directions related to the effects of CHX on bacteria in oral biofilms.
Keywords: CHX; adaptation; antiseptic; chlorhexidine; efflux pump; persistence; resistance; tolerance.
Figures

References
-
- Al-Ahmad A., Ameen H., Pelz K., Karygianni L., Wittmer A., Anderson A. C., et al. (2014). Antibiotic resistance and capacity for biofilm formation of different bacteria isolated from endodontic infections associated with root-filled teeth. J. Endod. 40 223–230. 10.1016/j.joen.2013.07.023 - DOI - PubMed
-
- Al-Ahmad A., Walankiewicz A., Hellwig E., Follo M., Tennert C., Wittmer A., et al. (2016). Photoinactivation using visible light plus water-filtered infrared-A (vis+wIRA) and chlorine e6 (Ce6) eradicates planktonic periodontal pathogens and subgingival biofilms. Front. Microbiol. 7:1900. 10.3389/fmicb.2016.01900 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

