Holm Oak Somatic Embryogenesis: Current Status and Future Perspectives
- PMID: 30967881
- PMCID: PMC6438927
- DOI: 10.3389/fpls.2019.00239
Holm Oak Somatic Embryogenesis: Current Status and Future Perspectives
Abstract
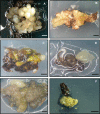
Quercus ilex (holm oak) is one of the most representative trees in the Mediterranean basin, but now the sustainability of its ecosystems is at serious risk due to the lack of natural regeneration and to the presence of a severe disease called oak decline that has caused the death of thousands of trees. The application of biotechnological tools, such as somatic embryogenesis, allows programs of genetic improvement of the species to be speeded up and helps in the conservation of its ecosystems. Somatic embryogenesis is currently considered one of the main biotechnological techniques that has demonstrated significant benefits when has applied to forest tree species, providing significant advantages such as mass propagation, genetic transformation, application of synthetic seed, and cryopreservation of elite genotypes. In this report, the state of the art of somatic embryogenesis in holm oak is reviewed. Factors affecting the induction (plant donor age, type of explant, or plant growth regulators) and maintenance and proliferation of the embryogenic cultures are summarized. Advances on the conversion of somatic embryos into plants and on the acclimatization of these plantlets, as well as the results obtained on the application of the genetic transformation and the cryopreservation procedures to holm oak embryogenic cultures, are also presented.
Keywords: Quercus ilex; cryopreservation; genetic transformation; oak decline; plant regeneration; somatic embryos.
Figures


References
-
- Anagnostakis S. L. (2001). The effect of multiple importations of pests and pathogens on a native tree. Biol. Invasions 3, 245–254. 10.1023/A:1015205005751 - DOI
-
- Ballester A., Corredoira E., Vieitez A. M. (2016). “Limitations of somatic embryogenesis in hardwood trees” in Vegetative pr opagation of forest trees. eds. Park Y. S., Bonga J. M., Moon H.-K. (Seoul: National Institute of Forest Science (Nifos)), 56–74. 10.1080/07352689.2018.1551122 - DOI
-
- Barakat A., DiLoreto D. S., Zhang Y., Smith C., Baier K., Powell W. A., et al. . (2009). Comparison of the transcriptomes of American chestnut (Castanea dentata) and Chinese chestnut (Castanea mollissima) in response to the chestnut blight infection. BMC Plant Biol. 9:51. 10.1186/1471-2229-9-51, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

