Engineering an Artificial T-Cell Stimulating Matrix for Immunotherapy
- PMID: 30968468
- PMCID: PMC8601018
- DOI: 10.1002/adma.201807359
Engineering an Artificial T-Cell Stimulating Matrix for Immunotherapy
Abstract
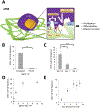
T cell therapies require the removal and culture of T cells ex vivo to expand several thousand-fold. However, these cells often lose the phenotype and cytotoxic functionality for mediating effective therapeutic responses. The extracellular matrix (ECM) has been used to preserve and augment cell phenotype; however, it has not been applied to cellular immunotherapies. Here, a hyaluronic acid (HA)-based hydrogel is engineered to present the two stimulatory signals required for T-cell activation-termed an artificial T-cell stimulating matrix (aTM). It is found that biophysical properties of the aTM-stimulatory ligand density, stiffness, and ECM proteins-potentiate T cell signaling and skew phenotype of both murine and human T cells. Importantly, the combination of the ECM environment and mechanically sensitive TCR signaling from the aTM results in a rapid and robust expansion of rare, antigen-specific CD8+ T cells. Adoptive transfer of these tumor-specific cells significantly suppresses tumor growth and improves animal survival compared with T cells stimulated by traditional methods. Beyond immediate immunotherapeutic applications, demonstrating the environment influences the cellular therapeutic product delineates the importance of the ECM and provides a case study of how to engineer ECM-mimetic materials for therapeutic immune stimulation in the future.
Keywords: T cell stimulation; adoptive T cell therapy; artificial matrix; extracellular matrix; hydrogel; immunotherapy; mechanotransduction.
© 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
- U54 CA210173/CA/NCI NIH HHS/United States
- #1s10OD020152-01A1/NH/NIH HHS/United States
- T32 CA130840/CA/NCI NIH HHS/United States
- DGE-1232825/National Science Foundation Graduate Research Fellowship
- ARCS foundation
- T32 CA153952/CA/NCI NIH HHS/United States
- R21 CA185819/CA/NCI NIH HHS/United States
- P41 EB028239/EB/NIBIB NIH HHS/United States
- 5R21CA185819-02/NH/NIH HHS/United States
- T32: 5T32CA153952-08/NH/NIH HHS/United States
- R01 EB029341/EB/NIBIB NIH HHS/United States
- T32 GM007309/GM/NIGMS NIH HHS/United States
- T32 GM136577/GM/NIGMS NIH HHS/United States
- U54 CA2101732/BC/NCI NIH HHS/United States
- 3T32GM007309/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

