A Critical Evaluation of Current Concepts in Cerebral Palsy
- PMID: 30968751
- PMCID: PMC7938766
- DOI: 10.1152/physiol.00054.2018
A Critical Evaluation of Current Concepts in Cerebral Palsy
Abstract
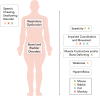
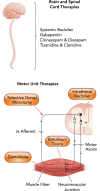
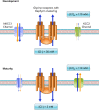
Spastic cerebral palsy (CP), despite the name, is not consistently identifiable by specific brain lesions. CP animal models focus on risk factors for development of CP, yet few reproduce the diagnostic symptoms. Animal models of CP must advance beyond risk factors to etiologies, including both the brain and spinal cord.
Figures
References
-
- Anstee QM, Knapp S, Maguire EP, Hosie AM, Thomas P, Mortensen M, Bhome R, Martinez A, Walker SE, Dixon CI, Ruparelia K, Montagnese S, Kuo YT, Herlihy A, Bell JD, Robinson I, Guerrini I, McQuillin A, Fisher EM, Ungless MA, Gurling HM, Morgan MY, Brown SD, Stephens DN, Belelli D, Lambert JJ, Smart TG, Thomas HC. Mutations in the Gabrb1 gene promote alcohol consumption through increased tonic inhibition. Nat Commun 4: 2816, 2013. doi:10.1038/ncomms3816. - DOI - PMC - PubMed
-
- Arneson CL, Durkin MS, Benedict RE, Kirby RS, Yeargin-Allsopp M, Van Naarden Braun K, Doernberg NS. Prevalence of cerebral palsy: Autism and Developmental Disabilities Monitoring Network, three sites, United States, 2004. Disabil Health J 2: 45–48, 2009. doi:10.1016/j.dhjo.2008.08.001. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

