Differential thermostability and response to cystic fibrosis transmembrane conductance regulator potentiators of human and mouse F508del-CFTR
- PMID: 30969810
- PMCID: PMC6689747
- DOI: 10.1152/ajplung.00034.2019
Differential thermostability and response to cystic fibrosis transmembrane conductance regulator potentiators of human and mouse F508del-CFTR
Abstract
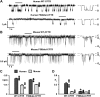
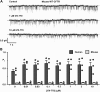
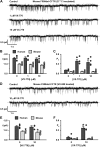
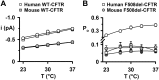
Cross-species comparative studies have highlighted differences between human and mouse cystic fibrosis transmembrane conductance regulator (CFTR), the epithelial Cl- channel defective in cystic fibrosis (CF). Here, we compare the impact of the most common CF mutation F508del on the function of human and mouse CFTR heterologously expressed in mammalian cells and their response to CFTR modulators using the iodide efflux and patch-clamp techniques. Once delivered to the plasma membrane, human F508del-CFTR exhibited a severe gating defect characterized by infrequent channel openings and was thermally unstable, deactivating within minutes at 37°C. By contrast, the F508del mutation was without effect on the gating pattern of mouse CFTR, and channel activity demonstrated thermostability at 37°C. Strikingly, at all concentrations tested, the clinically approved CFTR potentiator ivacaftor was without effect on the mouse F508del-CFTR Cl- channel. Moreover, eight CFTR potentiators, including ivacaftor, failed to generate CFTR-mediated iodide efflux from CHO cells expressing mouse F508del-CFTR. However, they all produced CFTR-mediated iodide efflux with human F508del-CFTR-expressing CHO cells, while fifteen CFTR correctors rescued the plasma membrane expression of both human and mouse F508del-CFTR. Interestingly, the CFTR potentiator genistein enhanced CFTR-mediated iodide efflux from CHO cells expressing either human or mouse F508del-CFTR, whereas it only potentiated human F508del-CFTR Cl- channels in cell-free membrane patches, suggesting that its action on mouse F508del-CFTR is indirect. Thus, the F508del mutation has distinct effects on human and mouse CFTR Cl- channels.
Keywords: CFTR chloride ion channel; CFTR potentiation; F508del-CFTR; cystic fibrosis; ivacaftor (VX-770).
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



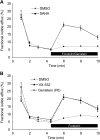

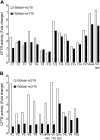

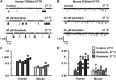
References
-
- Artigas P, Al’aref SJ, Hobart EA, Díaz LF, Sakaguchi M, Straw S, Andersen OS. 2,3-butanedione monoxime affects cystic fibrosis transmembrane conductance regulator channel function through phosphorylation-dependent and phosphorylation-independent mechanisms: the role of bilayer material properties. Mol Pharmacol 70: 2015–2026, 2006. doi:10.1124/mol.106.026070. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

