N-glycosylation-dependent regulation of hK2P17.1 currents
- PMID: 30969900
- PMCID: PMC6724686
- DOI: 10.1091/mbc.E18-10-0687
N-glycosylation-dependent regulation of hK2P17.1 currents
Abstract
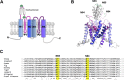
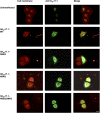
Two pore-domain potassium (K2P) channels mediate potassium background currents that stabilize the resting membrane potential and facilitate action potential repolarization. In the human heart, hK2P17.1 channels are predominantly expressed in the atria and Purkinje cells. Reduced atrial hK2P17.1 protein levels were described in patients with atrial fibrillation or heart failure. Genetic alterations in hK2P17.1 were associated with cardiac conduction disorders. Little is known about posttranslational modifications of hK2P17.1. Here, we characterized glycosylation of hK2P17.1 and investigated how glycosylation alters its surface expression and activity. Wild-type hK2P17.1 channels and channels lacking specific glycosylation sites were expressed in Xenopus laevis oocytes, HEK-293T cells, and HeLa cells. N-glycosylation was disrupted using N-glycosidase F and tunicamycin. hK2P17.1 expression and activity were assessed using immunoblot analysis and a two-electrode voltage clamp technique. Channel subunits of hK2P17.1 harbor two functional N-glycosylation sites at positions N65 and N94. In hemi-glycosylated hK2P17.1 channels, functionality and membrane trafficking remain preserved. Disruption of both N-glycosylation sites results in loss of hK2P17.1 currents, presumably caused by impaired surface expression. This study confirms diglycosylation of hK2P17.1 channel subunits and its pivotal role in cell-surface targeting. Our findings underline the functional relevance of N-glycosylation in biogenesis and membrane trafficking of ion channels.
Figures




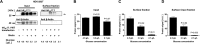

References
-
- Arnold K, Bordoli L, Kopp J, Schwede T. (2006). The SWISS-MODEL Workspace: a web-based environment for protein structure homology modelling. Bioinformatics , 195–201. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

