Structure and function of Spc42 coiled-coils in yeast centrosome assembly and duplication
- PMID: 30969903
- PMCID: PMC6724696
- DOI: 10.1091/mbc.E19-03-0167
Structure and function of Spc42 coiled-coils in yeast centrosome assembly and duplication
Abstract
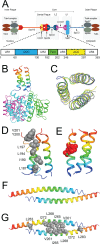
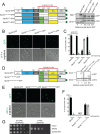
Centrosomes and spindle pole bodies (SPBs) are membraneless organelles whose duplication and assembly is necessary for bipolar mitotic spindle formation. The structural organization and functional roles of major proteins in these organelles can provide critical insights into cell division control. Spc42, a phosphoregulated protein with an N-terminal dimeric coiled-coil (DCC), assembles into a hexameric array at the budding yeast SPB core, where it functions as a scaffold for SPB assembly. Here, we present in vitro and in vivo data to elucidate the structural arrangement and biological roles of Spc42 elements. Crystal structures reveal details of two additional coiled-coils in Spc42: a central trimeric coiled-coil and a C-terminal antiparallel DCC. Contributions of the three Spc42 coiled-coils and adjacent undetermined regions to the formation of an ∼145 Å hexameric lattice in an in vitro lipid monolayer assay and to SPB duplication and assembly in vivo reveal structural and functional redundancy in Spc42 assembly. We propose an updated model that incorporates the inherent symmetry of these Spc42 elements into a lattice, and thereby establishes the observed sixfold symmetry. The implications of this model for the organization of the central SPB core layer are discussed.
Figures






Similar articles
-
The N-terminus of Sfi1 and yeast centrin Cdc31 provide the assembly site for a new spindle pole body.J Cell Biol. 2021 Mar 1;220(3):e202004196. doi: 10.1083/jcb.202004196. J Cell Biol. 2021. PMID: 33523111 Free PMC article.
-
Cdc28/Cdk1 regulates spindle pole body duplication through phosphorylation of Spc42 and Mps1.Dev Cell. 2004 Aug;7(2):263-74. doi: 10.1016/j.devcel.2004.07.006. Dev Cell. 2004. PMID: 15296722
-
Key phosphorylation events in Spc29 and Spc42 guide multiple steps of yeast centrosome duplication.Mol Biol Cell. 2018 Sep 15;29(19):2280-2291. doi: 10.1091/mbc.E18-05-0296. Epub 2018 Jul 25. Mol Biol Cell. 2018. PMID: 30044722 Free PMC article.
-
Lessons from yeast: the spindle pole body and the centrosome.Philos Trans R Soc Lond B Biol Sci. 2014 Sep 5;369(1650):20130456. doi: 10.1098/rstb.2013.0456. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 25047610 Free PMC article. Review.
-
Duplication of the Yeast Spindle Pole Body Once per Cell Cycle.Mol Cell Biol. 2016 Apr 15;36(9):1324-31. doi: 10.1128/MCB.00048-16. Print 2016 May. Mol Cell Biol. 2016. PMID: 26951196 Free PMC article. Review.
Cited by
-
Structural insights into the interplay between microtubule polymerases, γ-tubulin complexes and their receptors.Nat Commun. 2025 Jan 5;16(1):402. doi: 10.1038/s41467-024-55778-7. Nat Commun. 2025. PMID: 39757296 Free PMC article.
-
The core spindle pole body scaffold Ppc89 links the pericentrin orthologue Pcp1 to the fission yeast spindle pole body via an evolutionarily conserved interface.Mol Biol Cell. 2024 Aug 1;35(8):ar112. doi: 10.1091/mbc.E24-05-0220. Epub 2024 Jul 10. Mol Biol Cell. 2024. PMID: 38985524 Free PMC article.
-
CM1-driven assembly and activation of yeast γ-tubulin small complex underlies microtubule nucleation.Elife. 2021 May 5;10:e65168. doi: 10.7554/eLife.65168. Elife. 2021. PMID: 33949948 Free PMC article.
-
The N-terminus of Sfi1 and yeast centrin Cdc31 provide the assembly site for a new spindle pole body.J Cell Biol. 2021 Mar 1;220(3):e202004196. doi: 10.1083/jcb.202004196. J Cell Biol. 2021. PMID: 33523111 Free PMC article.
-
The Dictyostelium Centrosome.Cells. 2021 Oct 5;10(10):2657. doi: 10.3390/cells10102657. Cells. 2021. PMID: 34685637 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

