Precise staging of beetle horn formation in Trypoxylus dichotomus reveals the pleiotropic roles of doublesex depending on the spatiotemporal developmental contexts
- PMID: 30969957
- PMCID: PMC6457530
- DOI: 10.1371/journal.pgen.1008063
Precise staging of beetle horn formation in Trypoxylus dichotomus reveals the pleiotropic roles of doublesex depending on the spatiotemporal developmental contexts
Abstract
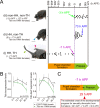
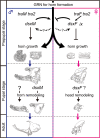
Many scarab beetles have sexually dimorphic exaggerated horns that are an evolutionary novelty. Since the shape, number, size, and location of horns are highly diverged within Scarabaeidae, beetle horns are an attractive model for studying the evolution of sexually dimorphic and novel traits. In beetles including the Japanese rhinoceros beetle Trypoxylus dichotomus, the sex differentiation gene doublesex (dsx) plays a crucial role in sexually dimorphic horn formation during larval-pupal development. However, knowledge of when and how dsx drives the gene regulatory network (GRN) for horn formation to form sexually dimorphic horns during development remains elusive. To address this issue, we identified a Trypoxylus-ortholog of the sex determination gene, transformer (tra), that regulates sex-specific splicing of the dsx pre-mRNA, and whose loss of function results in sex transformation. By knocking down tra function at multiple developmental timepoints during larval-pupal development, we estimated the onset when the sex-specific GRN for horn formation is driven. In addition, we also revealed that dsx regulates different aspects of morphogenetic activities during the prepupal and pupal developmental stages to form appropriate morphologies of pupal head and thoracic horn primordia as well as those of adult horns. Based on these findings, we discuss the evolutionary developmental background of sexually dimorphic trait growth in horned beetles.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
The role of doublesex in the evolution of exaggerated horns in the Japanese rhinoceros beetle.EMBO Rep. 2013 Jun;14(6):561-7. doi: 10.1038/embor.2013.50. Epub 2013 Apr 23. EMBO Rep. 2013. PMID: 23609854 Free PMC article.
-
Diversification of doublesex function underlies morph-, sex-, and species-specific development of beetle horns.Proc Natl Acad Sci U S A. 2012 Dec 11;109(50):20526-31. doi: 10.1073/pnas.1118589109. Epub 2012 Nov 26. Proc Natl Acad Sci U S A. 2012. PMID: 23184999 Free PMC article.
-
The Fat-Dachsous signaling pathway regulates growth of horns in Trypoxylus dichotomus, but does not affect horn allometry.J Insect Physiol. 2018 Feb-Mar;105:85-94. doi: 10.1016/j.jinsphys.2018.01.006. Epub 2018 Jan 31. J Insect Physiol. 2018. PMID: 29366850
-
Endocrine Control of Exaggerated Trait Growth in Rhinoceros Beetles.Integr Comp Biol. 2016 Aug;56(2):247-59. doi: 10.1093/icb/icw042. Epub 2016 Jun 1. Integr Comp Biol. 2016. PMID: 27252223 Review.
-
Recent advances in understanding horn formation in the Japanese rhinoceros beetle Trypoxylus dichotomus using next-generation sequencing technology.Curr Opin Insect Sci. 2022 Jun;51:100901. doi: 10.1016/j.cois.2022.100901. Epub 2022 Mar 14. Curr Opin Insect Sci. 2022. PMID: 35301164 Review.
Cited by
-
Phylogeny and biogeography of the Japanese rhinoceros beetle, Trypoxylus dichotomus (Coleoptera: Scarabaeidae) based on SNP markers.Ecol Evol. 2020 Dec 22;11(1):153-173. doi: 10.1002/ece3.6982. eCollection 2021 Jan. Ecol Evol. 2020. PMID: 33437420 Free PMC article.
-
Noncanonical function of the Sex lethal gene controls the protogyny phenotype in Drosophila melanogaster.Sci Rep. 2022 Jan 27;12(1):1455. doi: 10.1038/s41598-022-05147-5. Sci Rep. 2022. PMID: 35087103 Free PMC article.
-
Gene regulatory networks underlying the development and evolution of plasticity in horned beetles.Curr Opin Insect Sci. 2023 Dec;60:101114. doi: 10.1016/j.cois.2023.101114. Epub 2023 Sep 13. Curr Opin Insect Sci. 2023. PMID: 37709168 Free PMC article. Review.
-
Computational analyses decipher the primordial folding coding the 3D structure of the beetle horn.Sci Rep. 2021 Jan 13;11(1):1017. doi: 10.1038/s41598-020-79757-2. Sci Rep. 2021. PMID: 33441712 Free PMC article.
-
Post-embryonic tail development through molting of the freshwater shrimp Neocaridina denticulata.iScience. 2025 Jan 23;28(2):111885. doi: 10.1016/j.isci.2025.111885. eCollection 2025 Feb 21. iScience. 2025. PMID: 40051830 Free PMC article.
References
-
- Eberhard W.G., 1980. Horned beetles. Sci. Am. 242, 166–183. http://www.jstor.org/stable/24966285
-
- Siva-Jothy M.T., 1987. Mate securing tactics and the cost of fighting in the Japanese horned beetle, Allomyrina dichotoma L. (Scarabaeidae). J. Ethol. 5, 165–172. 10.1007/BF02349949 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

