Cytokinin inhibits cotton fiber initiation by disrupting PIN3a-mediated asymmetric accumulation of auxin in the ovule epidermis
- PMID: 30970146
- PMCID: PMC6598071
- DOI: 10.1093/jxb/erz162
Cytokinin inhibits cotton fiber initiation by disrupting PIN3a-mediated asymmetric accumulation of auxin in the ovule epidermis
Abstract
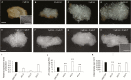
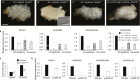
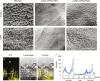
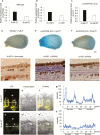
Auxin-dependent cell expansion is crucial for initiation of fiber cells in cotton (Gossypium hirsutum), which ultimately determines fiber yield and quality. However, the regulation of this process is far from being well understood. In this study, we demonstrate an antagonistic effect between cytokinin (CK) and auxin on cotton fiber initiation. In vitro and in planta experiments indicate that enhanced CK levels can reduce auxin accumulation in the ovule integument, which may account for the defects in the fiberless mutant xu142fl. In turn, supplementation with auxin can recover fiber growth of CK-treated ovules and mutant ovules. We further found that GhPIN3a is a key auxin transporter for fiber-cell initiation and is polarly localized to the plasma membranes of non-fiber cells, but not to those of fiber cells. This polar localization allows auxin to be transported within the ovule integument while specifically accumulating in fiber cells. We show that CKs antagonize the promotive effect of auxin on fiber cell initiation by undermining asymmetric accumulation of auxin in the ovule epidermis through down-regulation of GhPIN3a and disturbance of the polar localization of the protein.
Keywords: Auxin; GhPIN3a; cotton; cytokinin; fiber initiation; polar auxin transport.
© Society for Experimental Biology 2019.
Figures



Comment in
-
Optimization of polar distribution of GhPIN3a in the ovule epidermis improves cotton fiber development.J Exp Bot. 2019 Jun 28;70(12):3021-3023. doi: 10.1093/jxb/erz183. J Exp Bot. 2019. PMID: 31250905 Free PMC article.
References
-
- Beasley CA. 1973. Hormonal regulation of growth in unfertilized cotton ovules. Science 179, 1003–1005. - PubMed
-
- Beasley CA, Ting IP. 1973. The effects of plant growth substances on in vitro fiber development from fertilized cotton ovules. American Journal of Botany 60, 130–139.
-
- Beasley CA, Ting IP. 1974. Effects of plant growth substances on in vitro fiber development from unfertilized cotton ovules. American Journal of Botany 61, 188–194.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

