Anticancer Activities of Thymus vulgaris L. in Experimental Breast Carcinoma in Vivo and in Vitro
- PMID: 30970626
- PMCID: PMC6479806
- DOI: 10.3390/ijms20071749
Anticancer Activities of Thymus vulgaris L. in Experimental Breast Carcinoma in Vivo and in Vitro
Abstract
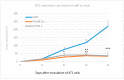
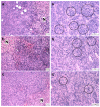
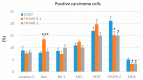
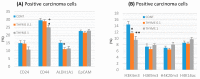
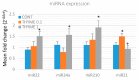
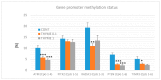
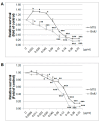
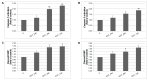
Naturally-occurring mixtures of phytochemicals present in plant foods are proposed to possess tumor-suppressive activities. In this work, we aimed to evaluate the antitumor effects of Thymus vulgaris L. in in vivo and in vitro mammary carcinoma models. Dried T. vulgaris (as haulm) was continuously administered at two concentrations of 0.1% and 1% in the diet in a chemically-induced rat mammary carcinomas model and a syngeneic 4T1 mouse model. After autopsy, histopathological and molecular analyses of rodent mammary carcinomas were performed. In addition, in vitro evaluations using MCF-7 and MDA-MB-231 cells were carried out. In mice, T. vulgaris at both doses reduced the volume of 4T1 tumors by 85% (0.1%) and 84% (1%) compared to the control, respectively. Moreover, treated tumors showed a substantial decrease in necrosis/tumor area ratio and mitotic activity index. In the rat model, T. vulgaris (1%) decreased the tumor frequency by 53% compared to the control. Analysis of the mechanisms of anticancer action included well-described and validated diagnostic and prognostic markers that are used in both clinical approach and preclinical research. In this regard, the analyses of treated rat carcinoma cells showed a CD44 and ALDH1A1 expression decrease and Bax expression increase. Malondialdehyde (MDA) levels and VEGFR-2 expression were decreased in rat carcinomas in both the T. vulgaris treated groups. Regarding the evaluations of epigenetic changes in rat tumors, we found a decrease in the lysine methylation status of H3K4me3 in both treated groups (H3K9m3, H4K20m3, and H4K16ac were not changed); up-regulations of miR22, miR34a, and miR210 expressions (only at higher doses); and significant reductions in the methylation status of four gene promoters-ATM serin/threonine kinase, also known as the NPAT gene (ATM); Ras-association domain family 1, isoform A (RASSF1); phosphatase and tensin homolog (PTEN); and tissue inhibitor of metalloproteinase-3 (TIMP3) (the paired-like homeodomain transcription factor (PITX2) promoter was not changed). In vitro study revealed the antiproliferative and proapoptotic effects of essential oils of T. vulgaris in MCF-7 and MDA-MB-231 cells (analyses of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) (MTS); 5-bromo-20-deoxyuridine (BrdU); cell cycle; annexin V/PI; caspase-3/7; Bcl-2; PARP; and mitochondrial membrane potential). T. vulgaris L. demonstrated significant chemopreventive and therapeutic activities against experimental breast carcinoma.
Keywords: MCF-7 cells; MDA-MB-231 cells; Thymus vulgaris; angiogenesis; apoptosis; cancer stem cells; cell proliferation; epigenetics; mammary carcinogenesis; predictive and preventive medicine; rat.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

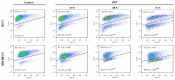

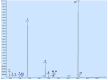
References
-
- Giacosa A., Barale R., Bavaresco L., Gatenby P., Gerbi V., Janssens J., Johnston B., Kas K., La Vecchia C., Mainguet P., et al. Cancer prevention in Europe: The Mediterranean diet as a protective choice. Eur. J. Cancer Prev. 2013;22:90–95. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

