The Potential Role of iNOS in Ovarian Cancer Progression and Chemoresistance
- PMID: 30970628
- PMCID: PMC6479373
- DOI: 10.3390/ijms20071751
The Potential Role of iNOS in Ovarian Cancer Progression and Chemoresistance
Abstract
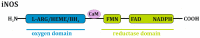
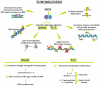
Inducible nitric oxide synthase (iNOS), the enzyme responsible for nitric oxide (NO) production, is not present in most cells under normal conditions. The expression of its mRNA, as well as its protein synthesis and full enzymatic activity, undergoes multilevel regulation including transcriptional and posttranscriptional mechanisms, the availability of iNOS substrate and cofactors and oxygen tension. However, in various malignant diseases, such as ovarian cancer, the intracellular mechanisms controlling iNOS are dysregulated, resulting in the permanent induction of iNOS expression and activation. The present review summarizes the multistaged processes occurring in normal cells that promote NO synthesis and focuses on factors regulating iNOS expression in ovarian cancer. The possible involvement of iNOS in the chemoresistance of ovarian cancer and its potential as a prognostic/predictive factor in the course of disease development are also reviewed. According to the available yet limited data, it is difficult to draw unequivocal conclusions on the pros and cons of iNOS in ovarian cancer. Most clinical data support the hypothesis that high levels of iNOS expression in ovarian tumors are associated with a greater risk of disease relapse and patient death. However, in vitro studies with various ovarian cancer cell lines indicate a correlation between a high level of iNOS expression and sensitivity to cisplatin.
Keywords: chemoresistance; iNOS; ovarian cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Pautz A., Art J., Hahn S., Nowag S., Voss C., Kleinert H. Nitric Oxide. Volume 23. Academic Press; Cambridge, MA, USA: 2010. Regulation of the expression of inducible nitric oxide synthase; pp. 75–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

