Polymers in Carbon Dots: A Review
- PMID: 30970747
- PMCID: PMC6432044
- DOI: 10.3390/polym9020067
Polymers in Carbon Dots: A Review
Abstract
Carbon dots (CDs) have been widely studied since their discovery in 2004 as a green substitute of the traditional quantum dots due to their excellent photoluminescence (PL) and high biocompatibility. Meanwhile, polymers have increasingly become an important component for both synthesis and modification of CDs to provide polymeric matrix and enhance their PL property. Furthermore, critical analysis of composites of CDs and polymers has not been available. Herein, in this review, we summarized the use of polymers in the synthesis and functionalization of CDs, and the applications of these CDs in various fields.
Keywords: carbon dots; composites; polymerization; polymers; surface modification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




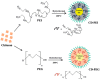
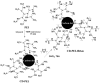


References
-
- Schulte J. Nanotechnology in environmental protection and pollution sustainable future, environmental cleanup and energy solutions. Sci. Technol. Adv. Mater. 2007;8:11. doi: 10.1016/j.stam.2006.11.019. - DOI
-
- Yang Y., Zheng Y., Cao W., Titov A., Hyvonen J., MandersJesse R., Xue J., Holloway P.H., Qian L. High-efficiency light-emitting devices based on quantum dots with tailored nanostructures. Nat. Photonics. 2015;9:259–266. doi: 10.1038/nphoton.2015.36. - DOI
-
- Mo Y.-M., Tang Y., Gao F., Yang J., Zhang Y.-M. Synthesis of fluorescent CdS quantum dots of tunable light emission with a new in situ produced capping agent. Ind. Eng. Chem. Res. 2012;51:5995–6000. doi: 10.1021/ie201826e. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

