Systematic Study of a Library of PDMAEMA-Based, Superparamagnetic Nano-Stars for the Transfection of CHO-K1 Cells
- PMID: 30970835
- PMCID: PMC6432303
- DOI: 10.3390/polym9050156
Systematic Study of a Library of PDMAEMA-Based, Superparamagnetic Nano-Stars for the Transfection of CHO-K1 Cells
Abstract
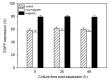
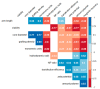
The introduction of the DNA into mammalian cells remains a challenge in gene delivery, particularly in vivo. Viral vectors are unmatched in their efficiency for gene delivery, but may trigger immune responses and cause severe side-reactions. Non-viral vectors are much less efficient. Recently, our group has suggested that a star-shaped structure improves and even transforms the gene delivery capability of synthetic polycations. In this contribution, this effect was systematically studied using a library of highly homogeneous, paramagnetic nano-star polycations with varied arm lengths and grafting densities. Gene delivery was conducted in CHO-K1 cells, using a plasmid encoding a green fluorescent reporter protein. Transfection efficiencies and cytotoxicities varied systematically with the nano-star architecture. The arm density was particularly important, with values of approximately 0.06 arms/nm² yielding the best results. In addition, a certain fraction of the cells became magnetic during transfection. The gene delivery potential of a nano-star and its ability to render the cells magnetic did not have any correlations. End-capping the polycation arms with di(ethylene glycol) methyl ether methacrylate (PDEGMA) significantly improved serum compatibility under transfection conditions; such nano-stars are potential candidates for future in vivo testing.
Keywords: ATRP; CHO cells; EGFP; PDEGMA; PDMAEMA; cellular uptake; gene delivery; magnetic nanoparticles; polycation; transfection.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures


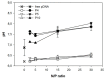
 ), magnetic cell fractions (
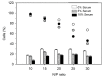
), magnetic cell fractions ( ), and viabilities (○) determined 26 h post transfection. Data represent mean ± S.E.M., n ≥ 3. In the case of the viabilities, statistically significant differences (p < 0.05) compared to mock transfected cells (97.6% ± 2.5%, n ≥ 30) are denoted by #.
), and viabilities (○) determined 26 h post transfection. Data represent mean ± S.E.M., n ≥ 3. In the case of the viabilities, statistically significant differences (p < 0.05) compared to mock transfected cells (97.6% ± 2.5%, n ≥ 30) are denoted by #.

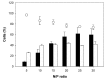
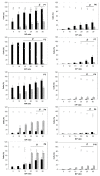
 ), magnetic cell fractions (
), magnetic cell fractions ( ), and viabilities (○) determined at 26 h post transfection. Data represent mean ± S.E.M., n ≥ 3.
), and viabilities (○) determined at 26 h post transfection. Data represent mean ± S.E.M., n ≥ 3.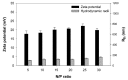
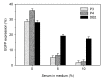
References
Grants and funding
LinkOut - more resources
Full Text Sources

