Preparation and Chromatographic Application of β-Cyclodextrin Molecularly Imprinted Microspheres for Paeoniflorin
- PMID: 30970892
- PMCID: PMC6431905
- DOI: 10.3390/polym9060214
Preparation and Chromatographic Application of β-Cyclodextrin Molecularly Imprinted Microspheres for Paeoniflorin
Abstract
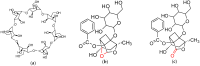
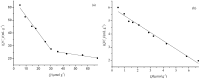
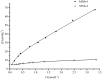
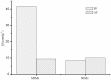
The application of molecular imprinting technology in the separation and purification of active ingredients in natural products was widely reported, but remains a challenge. Enrichment and separation are especially limited. A surface imprinting technique was reported to synthesize molecularly imprinted microspheres (MIMs) in this article. With paeoniflorin (PF) as the template molecule, β-cyclodextrin (β-CD) and acrylamide (AA) as the functional monomers, and poly(glycidyl methacrylate, GMA) microspheres (PGMA) as the backing material. MIMs have been characterized by FTIR and FESEM. Adsorption experiments indicated the adsorption capacity of MIMs was superior to those comparative non-imprinted microspheres (NIMs) and the binding isotherm of MIMs was in good agreement with the two-site binding model. The baseline separation of PF and its structural analogue albiflorin (AF) were achieved on the new MIMs packed column. MIMs showed good affinity and efficiency for separation of PF and AF compared with those comparative NIMs. The approach of fabricating MIMs is simple, rapid, and inexpensive, and may shed new light on the application of MIMs as a liquid chromatography stationary phase to separate and analyze PF and AF from the Red peony root extracts.
Keywords: chromatographic application; molecular imprinting; paeoniflorin; stationary phase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Kaneda M., Iitaka Y., Shibata S. Chemical studies on the oriental plant drugs-XXXIII: The absolute structures of paeoniflorin, albiflorin, oxypaeoniflorin and benzoylpaeoniflorin isolated from Chinese paeony root. Tetrahedron. 1972;28:4309–4317. doi: 10.1016/S0040-4020(01)88953-5. - DOI
-
- Sun R., Yi Y.P., Lv L.L., Zhang Z.P., Sun H., Liu G.Q. Effects of paeoniflorin on pathological changes in global brain ischemia model rats. China J. Chin. Mater. Med. 2007;32:2518–2522. - PubMed
-
- Rosen R. Herbal Skin Formulation. 7,235,265. U.S. Patent. 2007 Jun 26;
Grants and funding
LinkOut - more resources
Full Text Sources

