The Distribution of Nanoclay Particles at the Interface and Their Influence on the Microstructure Development and Rheological Properties of Reactively Processed Biodegradable Polylactide/Poly(butylene succinate) Blend Nanocomposites
- PMID: 30971028
- PMCID: PMC6418579
- DOI: 10.3390/polym9080350
The Distribution of Nanoclay Particles at the Interface and Their Influence on the Microstructure Development and Rheological Properties of Reactively Processed Biodegradable Polylactide/Poly(butylene succinate) Blend Nanocomposites
Abstract
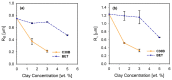
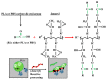
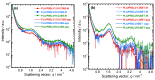
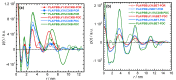
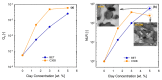
The present work investigates the distribution of nanoclay particles at the interface and their influence on the microstructure development and non-linear rheological properties of reactively processed biodegradable polylactide/poly(butylene succinate) blend nanocomposites. Two types of organoclays, one is more hydrophilic (Cloisite®30B (C30B)) and another one is more hydrophobic (BetsopaTM (BET)), were used at different concentrations. Surface and transmission electron microscopies were respectively used to study the blend morphology evolution and for probing the dispersion and distribution of nanoclay platelets within the blend matrix and at the interface. The results suggested that both organoclays tended to localize at the interface between the blend's two phases and encapsulate the dispersed poly(butylene succinate) phase, thereby suppressing coalescence. Using small angle X-ray scattering the probability of finding neighboring nanoclay particles in the blend matrix was calculated using the Generalized Indirect Fourier Transformation technique. Fourier Transform-rheology was utilized for quantifying nonlinear rheological responses and for correlating the extent of dispersion as well as the blend morphological evolution, for different organoclay loadings. The rheological responses were in good agreement with the X-ray scattering and electron microscopic results. It was revealed that C30B nanoparticles were more efficient in stabilizing the morphologies by evenly distributing at the interface. Nonlinear coefficient from FT-rheology was found to be more pronounced in case of blends filled with C30B, indicating better dispersion of C30B compare with BET which was in agreement with the SAXS results.
Keywords: morphology development; non-linear rheological properties; reactively compatibilized clay-containing PLA/PBS blends.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Ojijo V., Ray S.S. Processing strategies in bionanocomposites. Prog. Polym. Sci. 2013;38:1543–1589. doi: 10.1016/j.progpolymsci.2013.05.011. - DOI
-
- Ray S.S. Environmentally Friendly Polymer Nanocomposites. Elsevier; Amsterdam, The Netherlands: 2013.
-
- Odent J., Raquez J.-M., Leclère P., Lauro F., Dubois P. Crystallization-induced toughness of rubber-modified polylactide: Combined effects of biodegradable impact modifier and effective nucleating agent. Polym. Adv. Technol. 2015;26:814–822. doi: 10.1002/pat.3513. - DOI
-
- Ojijo V., Ray S.S. Super toughened biodegradable polylactide blends with non-linear copolymer interfacial architecture obtained via facile in situ reactive compatibilization. Polymer. 2015;80:1–17. doi: 10.1016/j.polymer.2015.10.038. - DOI
LinkOut - more resources
Full Text Sources

