Poly(3,4-ethylenedioxythiophene) (PEDOT) Derivatives: Innovative Conductive Polymers for Bioelectronics
- PMID: 30971030
- PMCID: PMC6418870
- DOI: 10.3390/polym9080354
Poly(3,4-ethylenedioxythiophene) (PEDOT) Derivatives: Innovative Conductive Polymers for Bioelectronics
Abstract
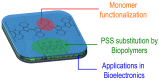
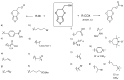
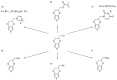
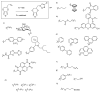
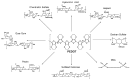
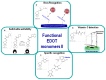
Poly(3,4-ethylenedioxythiophene)s are the conducting polymers (CP) with the biggest prospects in the field of bioelectronics due to their combination of characteristics (conductivity, stability, transparency and biocompatibility). The gold standard material is the commercially available poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS). However, in order to well connect the two fields of biology and electronics, PEDOT:PSS presents some limitations associated with its low (bio)functionality. In this review, we provide an insight into the synthesis and applications of innovative poly(ethylenedioxythiophene)-type materials for bioelectronics. First, we present a detailed analysis of the different synthetic routes to (bio)functional dioxythiophene monomer/polymer derivatives. Second, we focus on the preparation of PEDOT dispersions using different biopolymers and biomolecules as dopants and stabilizers. To finish, we review the applications of innovative PEDOT-type materials such as biocompatible conducting polymer layers, conducting hydrogels, biosensors, selective detachment of cells, scaffolds for tissue engineering, electrodes for electrophysiology, implantable electrodes, stimulation of neuronal cells or pan-bio electronics.
Keywords: PEDOT; bioelectronics; biopolymers; conducting polymers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

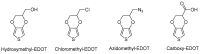




References
-
- Chiang C.K., Fincher C.R., Park Y.W., Heeger A.J., Shirakawa H., Louis E.J., Gau S.C., MacDiarmid A.G. Electrical Conductivity in Doped Polyacetylene. Phys. Rev. Lett. 1977;39:1098–1101. doi: 10.1103/PhysRevLett.39.1098. - DOI
-
- Wen Y., Xu J. Scientific Importance of Water-Processable PEDOT-PSS and Preparation, Challenge and New Application in Sensors of Its Film Electrode: A Review. J. Polym. Sci. A. 2017;55:1121–1150. doi: 10.1002/pola.28482. - DOI
-
- Berggren M., Richter-Dahlfors A. Organic Bioelectronics. Adv. Mater. 2007;19:3201–3213. doi: 10.1002/adma.200700419. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

