High-yield production of multiple O-methylated phenylpropanoids by the engineered Escherichia coli-Streptomyces cocultivation system
- PMID: 30971246
- PMCID: PMC6456975
- DOI: 10.1186/s12934-019-1118-9
High-yield production of multiple O-methylated phenylpropanoids by the engineered Escherichia coli-Streptomyces cocultivation system
Abstract
Background: O-Methylated phenylpropanoids, which are generally present in small amounts in plants, have improved or distinct biological activities and pharmacological properties as opposed to their unmethylated counterparts. Although microbial production could be a useful tool for the efficient and environment-friendly production of methylated phenylpropanoids, a high-yield microbial production of neither tri-methylated stilbenes nor di-/tri-methylated flavonoids has been achieved to date.
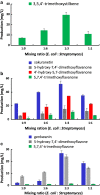
Results: A methyltransferase from Streptomyces avermitilis (SaOMT2), which has been known to possess 7-O-methylation activity toward several flavonoids, exhibited more diverse regiospecificity and catalyzed mono-, di-, and tri-methylation of stilbene, flavanone, and flavone when it was expressed in Streptomyces venezuelae. For the efficient production of multi-methylated phenylpropanoids, a cocultivation system was developed by employing engineered Escherichia coli strains producing pterostilbene, naringenin, and apigenin, respectively, along with SaOMT2-expressing S. venezuelae mutant. Consequently, high-yield microbial production of tri-methylated stilbenes and di-/tri-methylated flavonoids (including 3,5,4'-trimethoxystilbene, 5-hydroxy-7,4'-dimethoxyflavanone, 4'-hydroxy-5,7-dimethoxyflavanone, 5,7,4'-trimethoxyflavanone, 5-hydroxy-7,4'-dimethoxyflavone, and 5,7,4'-trimethoxyflavone) has been demonstrated for the first time.
Conclusions: This cocultivation system based on the phenylpropanoid-producing E. coli and SaOMT2-expressing S. venezuelae provides an efficient tool for producing scarce and potentially valuable multi-methylated phenylpropanoids and will enable further development of these compounds as pharmaceuticals and nutraceuticals.
Keywords: E. coli–Streptomyces cocultivation; O-methylated phenylpropanoids; O-methyltransferase.
Conflict of interest statement
Provisional patent applications covering this work have been filed.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

