Cbp-dependent histone acetylation mediates axon regeneration induced by environmental enrichment in rodent spinal cord injury models
- PMID: 30971452
- PMCID: PMC7355732
- DOI: 10.1126/scitranslmed.aaw2064
Cbp-dependent histone acetylation mediates axon regeneration induced by environmental enrichment in rodent spinal cord injury models
Abstract
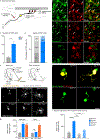
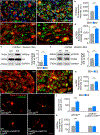
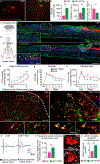
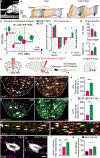
After a spinal cord injury, axons fail to regenerate in the adult mammalian central nervous system, leading to permanent deficits in sensory and motor functions. Increasing neuronal activity after an injury using electrical stimulation or rehabilitation can enhance neuronal plasticity and result in some degree of recovery; however, the underlying mechanisms remain poorly understood. We found that placing mice in an enriched environment before an injury enhanced the activity of proprioceptive dorsal root ganglion neurons, leading to a lasting increase in their regenerative potential. This effect was dependent on Creb-binding protein (Cbp)-mediated histone acetylation, which increased the expression of genes associated with the regenerative program. Intraperitoneal delivery of a small-molecule activator of Cbp at clinically relevant times promoted regeneration and sprouting of sensory and motor axons, as well as recovery of sensory and motor functions in both the mouse and rat model of spinal cord injury. Our findings showed that the increased regenerative capacity induced by enhancing neuronal activity is mediated by epigenetic reprogramming in rodent models of spinal cord injury. Understanding the mechanisms underlying activity-dependent neuronal plasticity led to the identification of potential molecular targets for improving recovery after spinal cord injury.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Competing interests:
LDFM is the director of the company Research Devices Ltd, but no conflicts of interest arise related to the work reported in this study. GC is a founder and shareholder of the company GTXmedical, but its activity is not directly linked to the interventions reported in this study. TKK holds the following patents related to CSP-TTK21. (a) International Patent: PCT/IN2008/000632. Title of the invention: Intrisically Fluorescent Carbon Nanospheres and a Process thereof. (b) US Patent WO2013160885 A1, US20150119466, European Patent EP2841111 A1. Title of the invention: Nanosphere-Histone Acetyltransferase (HAT) Activator Composition. process and methods thereof. (c) USS Patent:US93145399B2. Title of the invention: Nanosphere-histone acetyltransferase (HAT) activator composition, process and methods thereof. Otherwise the authors declare no competing interests.
Figures


References
-
- Sofroniew MV, Dissecting spinal cord regeneration. Nature 557, 343–350 (2018). - PubMed
-
- He Z, Jin Y, Intrinsic Control of Axon Regeneration. Neuron 90, 437–451 (2016). - PubMed
-
- Di Giovanni S, Molecular targets for axon regeneration: focus on the intrinsic pathways. Expert Opin Ther Targets 13, 1387–1398 (2009). - PubMed
-
- Plunet W, Kwon BK, Tetzlaff W, Promoting axonal regeneration in the central nervous system by enhancing the cell body response to axotomy. J Neurosci Res 68, 1–6 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

