Levocarnitine Improves AlCl3-Induced Spatial Working Memory Impairment in Swiss albino Mice
- PMID: 30971884
- PMCID: PMC6444114
- DOI: 10.3389/fnins.2019.00278
Levocarnitine Improves AlCl3-Induced Spatial Working Memory Impairment in Swiss albino Mice
Abstract
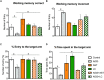
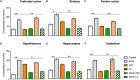
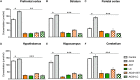
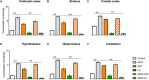
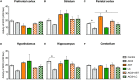
Background: Aluminum, a neurotoxic substance, causes oxidative stress induced-neurodegenerative diseases. Several lines of evidence suggest that levocarnitine has an antioxidant effect and also plays an important role in beta-oxidation of fatty acids. However, the role of levocarnitine in aluminum-induced neurotoxicity has not been well documented. Here we aimed to investigate the effect of levocarnitine on aluminum chloride (AlCl3)-induced oxidative stress and memory dysfunction. Methods: Male Swiss albino mice (n = 30) were treated with either control (saline) or AlCl3 or AlCl3 plus levocarnitine or levocarnitine or astaxanthin plus AlCl3 or astaxanthin alone. The spatial working memory was determined by radial arm maze (RAM). In addition, we measured the lipid peroxidation (MDA), glutathione (GSH), advanced oxidation of protein products (AOPP), nitric oxide (NO) and activity of superoxide dismutase (SOD) in the various brain regions including prefrontal cortex (PFC), striatum (ST), parietal cortex (PC), hippocampus (HIP) hypothalamus (HT) and cerebellum (CB). We used astaxanthin as a standard antioxidant to compare the antioxidant activity of levocarnitine. Results: The RAM data showed that AlCl3 treatment (50 mg/kg) for 2 weeks resulted in a significant deficit in spatial learning in mice. Moreover, aluminum exposure significantly (p < 0.05) increased the level of oxidative stress markers such as MDA, GSH, AOPP and NO in the various brain regions compared to the controls. In addition, combined administration of levocarnitine and AlCl3 significantly (p < 0.05) lowered the MDA, AOPP, GSH and NO levels in mice. Conclusion: Our results demonstrate that levocarnitine could serve as a potential therapeutic agent in the treatment of oxidative stress associated diseases as well as in memory impairment.
Keywords: Alzheimer’s disease; antioxidants; levocarnitine; neurotoxicity; oxidative stress markers; working memory.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

