Cyto- and Myelo-Architecture of the Amygdaloid Complex of the Common Marmoset Monkey (Callithrix jacchus)
- PMID: 30971903
- PMCID: PMC6446959
- DOI: 10.3389/fnana.2019.00036
Cyto- and Myelo-Architecture of the Amygdaloid Complex of the Common Marmoset Monkey (Callithrix jacchus)
Abstract
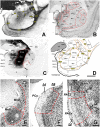
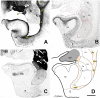
The amygdaloid complex (AC) is a heterogeneous aggregate of nuclei located in the rostromedial region of the temporal lobe. In addition to being partly connected among themselves, the AC nuclei are strongly interconnected with the cerebral cortex, striatum, basal forebrain, hypothalamus and brainstem. Animal and human functional studies have established that the AC is a central hub of the neuronal networks supporting emotional responsivity, particularly its negative/aversive components. Dysfunction of AC circuits in humans has been implicated in anxiety, depression, schizophrenia and bipolar disorder. The small New-World marmoset monkey (Callithrix jacchus) has recently become a key model for neuroscience research. However, the nuclear and fiber tract organization of marmoset AC has not been examined in detail. Thus, the extent to which it can be compared to the AC of Old-World (human and macaque) primates is yet unclear. Here, using Nissl and acetylcholinesterase (AChE) histochemical stains as a reference, we analyzed the cytoarchitecture and nuclear parcellation of the marmoset AC. In addition, given the increasing relevance of tractographic localization for high-resolution in vivo imaging studies in non-human primates, we also identified the myelin fiber tracts present within and around the AC as revealed by the Gallyas method. The present study provides a detailed atlas of marmoset AC. Moreover, it reveals that, despite phylogenetic distance and brain size differences, every nucleus and myelinated axon bundle described in human and macaque studies can be confidently recognized in marmosets.
Keywords: amygdaloid complex; marmoset Callithrix jacchus; myelin staining; nuclear division; tractography.
Figures




References
-
- Amaral D. G., Price J. L., Pitkanen A., Carmichael S. T. (1992). “Anatomical organization of the primate amygdaloid complex,” in The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfunction, ed. Aggleton J. P. (New York, NY: Wiley-Liss; ), 1–66.
-
- Avendaño C., Reinoso-Suarez F. (1975). Atlas Estereotáxico de la Amígdala, el Hipotálamo y la Región Preóptica del Gato. Servicio de publicaciones de la Universidad Autónoma de Madrid.
LinkOut - more resources
Full Text Sources

